What Is on Your HPLC Particle? A Look at Stationary Phase Chemistry Synthesis
The traditional bonding techniques used to manufacture silica-particle-based HPLC stationary phases provide many benefits, but can emerging technologies tackle unmet needs? We assess current approaches and the potential for improvements.
In 1973, Waters Corporation launched the first commercial 10 mm particle C18 column using a bonded monofunctional silane (mBondapak C18). Almost 50 years later, the C18 phase is still the reigning champion in the reversed-phase liquid chromatography (RPLC) arena, and many chromatography companies are still bonding using the same or a very similar synthetic approach. Several innovative bonding chemistries have been developed over the years to mitigate some of the common problems associated with the traditional C18 bonding approaches. These may include low and high pH stability, undesirable silanol activity, and a lack of polar retention. Nevertheless, particle technologies based on silica have received the most attention due to their undisputed chromatographic benefits. Silica supports offer high mechanical strength, allowing the formation of packed beds that are stable for long periods under high operating pressures. Controllable surface area, diversity in particle morphology, and higher efficiency values when compared to other support materials are just some of the advantages of silica-based columns. Advances in platforms that drift away from legacy products in terms of both the solid supports and in device architecture are trending in the literature. Some of these areas of interest include monoliths, open tubular columns (OTCs), microchip based columns, metal-organic frameworks (MOFs), and overall column and instrument miniaturization. Such developments will challenge traditional approaches toward surface chemistry modification. This article will review historical bonding techniques still in use for manufacturing HPLC stationary phases today, and also examine some emerging technologies that may be able to tackle unmet needs in novel platforms and phase construction.
Various advances in silica manufacturing have paved the way for modern chromatography. Since their inception in the 1970s, superficially porous particles (SPPs) have offered good performance and are indispensable in high-speed high performance liquid chromatography (HPLC) (1). Recent trends show the preference towards using SPP, especially in emerging markets such as cannabis (2); nevertheless, conventional particle sizes are still beneficial due to their higher surface area and their high loadability required for preparative scale applications. These advancements on solid supports have contributed great benefits to every industry where chromatography takes place, but novel approaches to functionalization of such platforms has remained stagnant for decades.
According to the United States Pharmacopeia (USP), there are 858 C18 liquid chromatography phases registered under code L1 (octadecylsilane chemically bonded to porous silica or ceramic microplates, 1.5 to 10 μm in diameter, or a monolithic rod) (3). The vast amount of commercially available C18 columns are constructed on a wide range of solid supports, and may exhibit ancillary options such as endcapping and aqueous compatibility (AQ). They can also be mixed with other RPLC phases. Although silica particle manufacturing has become more normalized in the last few decades, these subtle differences in commercial C18 make it extremely difficult for the novice, and even the seasoned chromatographer, to “grab a column and go” for a given application.
Silica is an amorphous polymer of silicon and oxygen. This polymer’s surface contains reactive silanols (Si-OH) that offer a number of possibilities for the synthesis of chemically-bonded phases. Organosilanes have been used as early as the 1950s to functionalize filter paper for the separation of steroids (4). In 1973, Locke correctly predicted that organosilanes would transform HPLC columns via chemically-bonded phases, mentioning that polymerization of silanes would be the synthetic route taken in general with chemical reactions being carried out to produce a primary organosilane layer (5). At the same time, he hoped for new developments in bonding stationary phases through the introduction of specific groups onto the organosilane bonding reagents; however, almost half a century later, organosilanes are still being grafted onto silica particles via the same chemistry described back then. Although new synthetic approaches have been applied to reversed-phase ligands, the bulk of the commercially available phases are still manufactured via conventional methods (6).
C18 Ligand Chemistries
Using reactions developed by John Speier at Dow Corning, Waters’ scientists successfully synthesized octadecyldimethylchlorosilane (ODS), leading to the first commercial monomeric-bonded C18 column in 1973 (7). Since then, traditional bondings of reversed phases use a monofunctional silane in order to maximize ligand coverage and avoid unwanted polymerization that could affect batch-to-batch reproducibility. A typical monofunctional silane will yield a ligand density of around 3-4 µmol/m2 under optimal bonding conditions, leaving behind up to 50% of the original amount of silanols based on an average of 8 µmol/m2 on a typical silica surface. HPLC phases labeled as “end-capped,” “maximum coverage,” or “high density” still abide by this maxima, and any residual silanol would still be able to interact with analytes, contributing to the overall adsorptive properties of the bonded phase.
Over the years, traditionally-bonded phases have suffered from disadvantages tied to the use of silica as a solid platform. The tethering of the ligand to the silica surface is subject to hydrolytic cleavage at pH < 2, leading to loss of bonded ligand while silica particles are prone to dissolution at pH > 8. However, several bonding chemistries have been developed to circumvent such disadvantages, and numerous commercially-available phases are able to mitigate these problem areas.
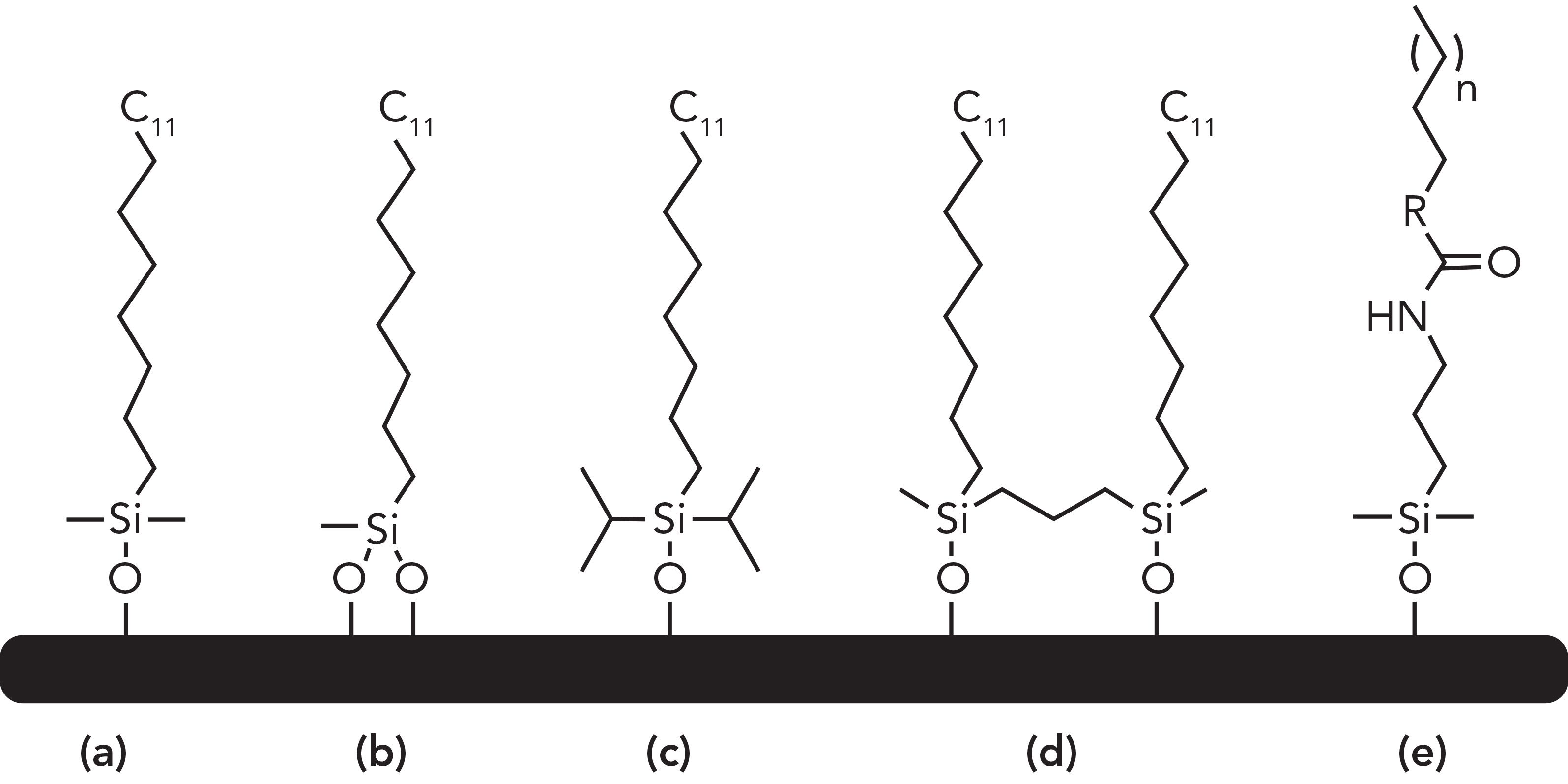
Figure 1 shows some of the innovative bonding constructs for several C18 phases. Element (a) shows a conventional monofunctional octadecylsilane and the most common phase available on the market. Element (b) shows a difunctional silane that is formed when a dichloro- or a dialkoxysilane is chemically bonded to silica; this double attachment is thought to increase the stability of the ligand at lower pH values and decrease phase bleed. Some manufacturers claim to use trifunctional silanes (not shown), but the exact control of tridentate reactions with the silica surface is still up to debate, and may lead to lower reproducibility of manufacturing. Element (c) shows a “bulky” silane where an isopropyl or isobutyl group hinders the siloxane linkage from hydrolytic cleavage at low pH values (8). Element (d) shows a “bridged” phase, where a bidentate organosilane is grafted on a silica particle, better shielding the surface from dissolution at a high pH. This latter bridging (crosslinking) technology can extend from one to several protective organic and inorganic layers, rendering high stability to the phase overall (9). This is not to be confused with hybrid silica particles where the organic moiety is a main component of the particle construction, and not just surface functionalization (10).
Figure 1: (a–e) A variety of commercial C18 ligands.
Element (e) in Figure 1 shows an alkyl chain much like its C18 counterparts, but contains a polar group intrinsic to the chain (amide, urea, and carbamate). These polar-embedded groups (PEG) have led to a new class of phases that offer some surface silanol shielding and additional polar retention (11). The polar-embedded moiety yields good peak shape towards basic analytes while making the phase compatible in 100% aqueous mobile phases without the “dewetting” effect. These polar functionalities can be obtained via a single step, using a pre-formed PEG containing silylating ligand directly onto silica, or a multi-step process, as outlined in Figure 2 (12). Amines and hydroxyl groups react with acid chlorides to yield amides and esters respectively; however, since ester groups are more unstable at low pH, amide linkages have been the preferred one among HPLC phases, along with sulfonamides (13). However, due to their toxicities, high reactivities, and non-selective behaviors, acid chlorides are rarely used in amide coupling reactions currently. Instead, several peptide-coupling reagents have been developed over the years to fulfill the safe and efficient processes required in drug development, and eventually applied towards manufacturing HPLC phases. Some of these popular reagents are highlighted in the following manuscripts for the construction of chiral and achiral ligands: 1,1´-carbonyldiimidazole (CDI) (14), hexafluorophosphate azabenzotriazole tetramethyl uronium (HATU) (15), 1-hydroxybenzotriazole (HOBT) (16), and N-Ethyl-N´-(3-dimethylaminopropyl)carbodiimide (EDC) (17).
Figure 2: Typical “polar embedding” functionalities found across HPLC phases.
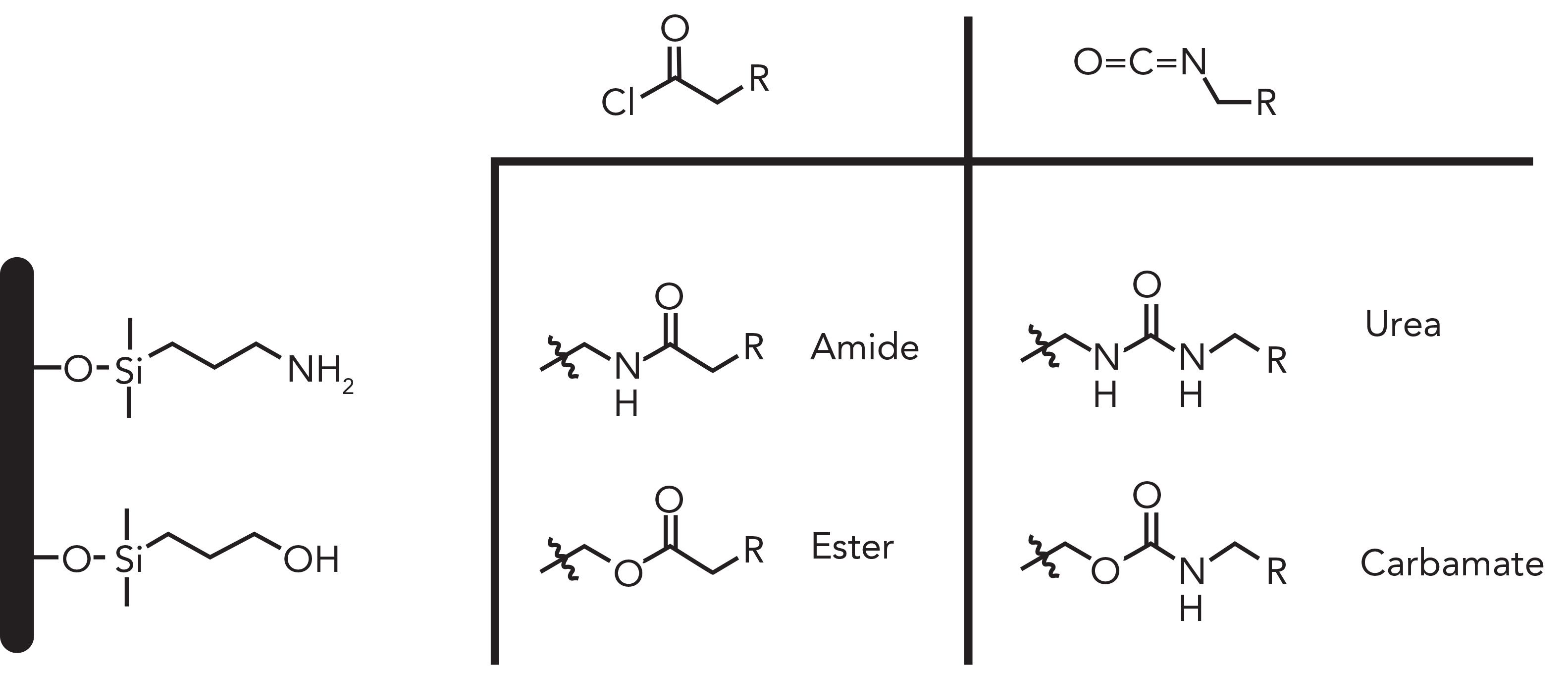
The other major categories of polar-embedded groups are ureas and carbamates. They are obtained by reacting isocyanates with primary amines and alcohols respectively. These functional groups provide additional polar interactions as hydrogen bond acceptors and less ionic interactions when compared to alkyl phases, leading to selectivity differences for polarizable compounds while improving peak shape of basic analytes (18). Additionally, carbamate linkages have been used specifically in the derivatization of chiral selectors such as Pirkle type (19) and polysaccharides due to the abundance of hydroxyl groups while enhancing their chiral recognition (20).
Silanization Chemistries
While the diversity of column chemistries for all types of separation modes is never ending, the chemistry of ligand attachment or grafting is not nearly as varied. Chlorosilanes, alkoxysilanes, and silazanes have been the workhorses of silica functionalization. They react through hydrolysis, condensation, and polymerization reactions, where a new siloxane bond (Si-O-Si) forms while yielding a small molecule, typically hydrochloride gas, diethylamine, methanol, ethanol, or water. The result of reacting an organosilane with silica’s surface is not only the bridging between organic and inorganic materials, but also is what imparts the main mode of separation to each stationary phase.
The advance of silicon-related technologies in material science has driven silane ligand synthesis. However, recent acquisitions of two major silane manufacturing companies leads to uncertainty of the current silane portfolio (21,22). Silane synthesis, although straightforward, suffers from one caveat: purification. Flash column chromatography is the method of choice when purifying a newly-synthesized compound from a mixture. However, a desired chlorosilane or alkoxysilane may permanently bind to the silica gel, leading to poor recoveries. Although some protocols exist to passivate silica gel from interacting with the silane ligand (23), the bulk of the reactive organosilane purification have relied on simple distillation. Such an approach leads to the confinement of commercially available ligands to a specific molecular weight range and containing functional groups that are thermally stable enough to endure purification by this technique.
An evolutionary bonding technology uses hydrosilanes, which Pesek developed through the development and the application of hydrosilylation chemistry in the production of HPLC stationary phases (24). The “Type C” silica possesses silica hydride (Si-H) at the surface of the particle and lacks the negative effects of silanols found on Type A and Type B silica. In order to functionalize this surface, a terminal alkyne or alkene will undergo hydrosilylation with Si-H in the presence of a platinum metal catalyst, leading to a phase with improved resistance to conditions that may cause hydrolysis in Type B silica columns. This is partially due to the much more hydrophobic surface offered by the silicon hydride moieties versus the usual hydrophilic silanol (25).
Figure 3 displays the reaction outcomes of alkene and alkyne hydrosilylation of a hydride-terminated particle. Alkynes may undergo a double hydrosilylation resulting in a bidentate attachment onto the surface, although most of the ligand will be singly attached via silicon-carbon double bond (Si=C) due to steric hindrance (26). The presence of hydrides, or the lack of silanols (<5%), and the strong Si-C or Si=C resulting from hydrosilylation is what gives these phases their chemically stability and interesting selectivity for chromatographic applications. Several metal catalysts have been used throughout the years but platinum remains the gold standard. Hexachloroplatinic acid (H2PtCl6), also known as Speier’s catalyst, and later the silicone-soluble Karstedt’s catalyst was adopted for this type of chemistry (27).
Figure 3: Examples of hydrosilylation reactions on hydride terminated support.
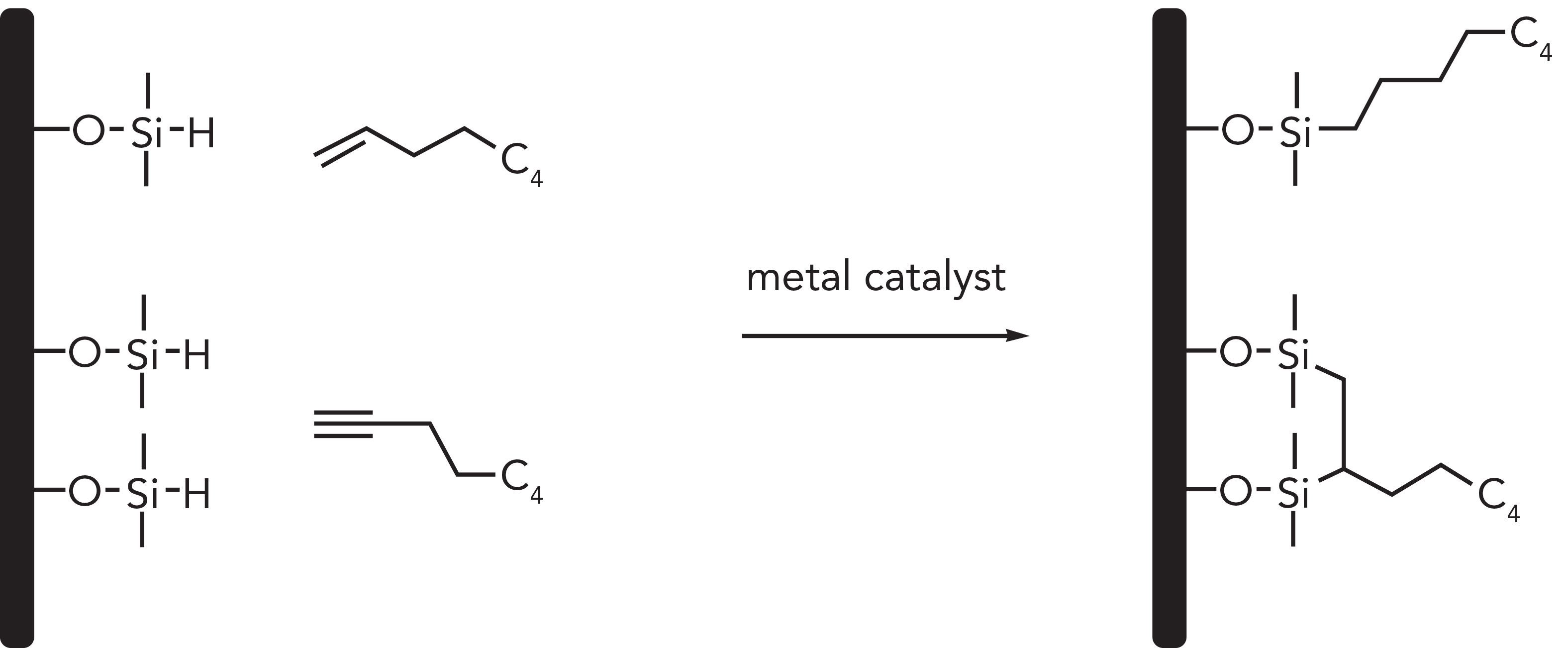
A look into the hydrosilylation platform synthesis leads to some unanswered questions and challenges. The first challenge is regarding the complete removal of the platinum reagent after the bonding reaction. The catalyst may form colloidal platinum that could deposit deep inside the particle making it almost impossible to remove; therefore, defeating the purpose of using Type B silica as the very first solid support (28). The second challenge is the catalyst’s selective functional group tolerance, leaving the weak anion-exchange and many mixed-mode phases out of the hydrosilylation platform (29). The third challenge, which it is still subject to debate, is whether the coverage of Si-H is enough (~95%), hindering as many surface silanol and the inertness of such moiety during the lifetime of the column (30,31). The last challenge is phase availability, and the difficulty of finding equivalent columns by other manufacturers (32).
Functionalization of Novel Solid Supports
The most common formats for analytical columns remain 4.6 mm and 2.1 mm i.d. dimensions, although smaller, capillary-size columns have found a place within the HPLC arena, especially for complex biological applications. Capillary (0.3–0.5 mm i.d.) and nano formats (0.075–0.1 mm i.d.) have become more popular in the last few years, and many vendors offer a decent variety of stationary phases in these dimensions. The sub-millimeter internal diameter columns are packed with functionalized silica particles via slurry methods similar to their larger dimension counterparts; nevertheless, this process has shown to be challenging, and much research has been devoted into this field (33,34). As an alternative, other phase supports, including monoliths and pillar arrays, have gained traction in recent years, and although both of them promise either lower backpressure or higher efficiencies than the particle packed beds (35), very little has been mentioned in regards to the pragmatic functionalization of such formats.
Monolithic beds are usually created in situ by free radical polymerization of monomers in the presence of porogens. Although thermal polymerization is a viable option, temperature fluctuations can occur in the confined spaces of the fluidic path, altering bed homogeneity along the column (36). Monomers can be organic (styrene, or acrylate-based) or siliceous in nature and offer the right amount of synthetic handles to permit surface functionalization. However, due to the nature of the inorganic monolith requiring a high temperature calcination step during its manufacturing, the appropriate functionalization must take place in situ as well. Hilder and co-workers developed a flow method for the ODS grafting on a 100 x 4.6 mm i.d. silica monolith, and its performance benchmarked against commercial C18 monolithic and a particle packed column (37). Since then, similar flow-like protocols have been applied to other column dimensions; however, slow reagent flow rates (mL/min) and high temperatures are still needed to graft a common C18 ligand, leading to bonding times of up to 24 h for a single analytical size column (38). Organic-based monoliths offer a wider variety of synthetic functionalization that are out of the scope of this article; however, their pervious behavior towards organic solvents and their hindered mass transfer kinetics for small molecules result in the chromatographic preference towards silica-based monoliths. For more information, El Rassi and co-workers recently published a review discussing several post-polymerization functionalization strategies (39).
Another column format involves microfabricated devices with microfluidic channels. Sepaniak and associates describe the functionalization of a pillar array architecture, which includes submerging the silicon oxide layers of the pillars in pure octadecyltrichlorosilane (OTS) and heating it to 170 oC for 2 h (40). On the other hand, De Malsche describes a flow method to functionalize the silica porous layer of radially elongated pillars (REP) where a solution of ODS is infused under 40 bar of pressure overnight. Although this method successfully grafted a C18 phase, the process only allows for a single “column” functionalization in about a 24-h window (41).
As novel formats show promising chromatographic benefits, whether they are open tubular columns, chip-based platforms, or overall miniaturization of the column compartment, the grafting protocols must deviate from legacy silanization chemistry to facilitate their large scale manufacturing while securing low cost and high batch-to-batch reproducibility. Vendors are currently able to manufacture functionalized silica in kilogram scale within 24 h, which can be used to pack hundreds or maybe even thousands of conventional analytical or capillary size columns. New solid supports and platforms such as metal-organic frameworks will obligate scientists to figure out interesting ways to functionalize them, given their lack of attachment points while at the same time widening their applicability in separation science (42).
Conclusion
Neue wrote that, when compared to gas chromatography (GC), variety in HPLC stationary phases is not necessary since scientists have control over the mobile phase composition, which is a powerful tool over the selectivity of the separation (43). However, we are seeing an influx of novel stationary phases in the literature and the market, especially in the mixed-mode arena, to fulfill wider customer demand for solutions. Many innovative chemistries have been designed to tackle the common problems of reversed-phase associated with silica as solid support from bulky silanes to polar embedded ones; nevertheless, with the exception of hydrosilylation platform, the actual silanization technique has remained largely the same for almost half a century. Miniaturized chromatography systems are already on the market, but the bulk of surface modification processes cannot be adapted into these new formats, especially at the rate of customer needs. Flow functionalization has shown to be an alternative to conventional particle bonding methods but reactions times per column manufacturing remain excessively high. Historically, silanization techniques have been developed by both academia and the silicon industry; furthermore, these new methods are readily available in the literature and require metal-free conditions, room temperature, and faster kinetics. It is time for chromatography firms to implement such innovative grafting protocols.
References
- J. Kirkland, Anal. Chem. 43(12), 36A–48A (1971).
- V. D’Atri, S. Fekete, A. Clarke, J.-L. Veuthey, and D. Guillarme, Anal. Chem. 91(1), 210–239 (2019).
- United States Pharmacopeia, USP 34 NF 29, 982–983 (2011).
- A. Zaffaroni, R.B. Burton, and E.H. Keutmann, Science 111(2871),6–8 (1950)..
- D.C. Locke, J. Chromatogr A 11(3), 120–128 (1973).
- S.H. Lee, J.S. Kang, and D. Kim, Materials 11 (12), 2557 (2018).
- U.D. Neue, Waters Col. Inter. 6(4), 13–15 (1997).
- J.J. Kirkland, J.L. Glajch, and R.D. Farlee, Anal. Chem. 61(1), 2–11 (1989).
- US Patent US 8,658,038 B2.
- T.H. Walter, LCGC North Am. 37 (7), 436–442 (2019).
- T.L. Ascah, and B. Feibush, J. Chromatogr. A 506, 357–369 (1990).
- M.R. Euerby, and P. Petersson, J. Chromatogr. A 1088(1), 1–15 (2005).
- X. Liu, A. Bordunov, M. Tracy, R. Slingsby, N. Avdalovic, and C. Pohl, J. Chromatogr. A 1119(1–2), 120–127 (2006).
- Y. Zhang, R. Lu, M. Chen, S. Zhou, D. Zhang, H. Han, M. Zhang, and H. Qiu, J. Chromatogr. A 1626, 461366 (2020).
- Y. Wang, D. Liu, Y. Zhang, Y. Tang, J. Zhao, and B. Shen, Symmetry 10(12), 704 (2018).
- H. Aral, K.S. Çelik, R. Altındağ, and T. Aral, Talanta 174, 703-714 (2017).
- C.J. Welch, J.O. DaSilva, J. Nti-Gyabaah, F. Antia, K. Goklen, and R. Boyd, J. Chromatogr. A 1101(1), 204–213 (2006).
- J. Mckenzie, D.S. Bell, H. Cramer, and C. Aurand, LCGC North Am. 32(9), 704–717 (2014).
- K.M. Kacprzak, and W. Lindner, J. Sep. Sci. 34(18), 2391–6 (2011).
- Y. Okamoto, and E. Yashima, Angew. Chem. Inter. Ed. 37(8), 1020–1043 (1998).
- M. McCoy, Chem. Eng. News 98(17), (2020).
- M. McCoy, Chem. Eng. News 97(29), (2019).
- Y. Barness, O. Gershevitz, M. Sekar, and C. N. Sukenik, Langmuir 16(1), 247–251 (2000).
- J.E. Sandoval, and J.J. Pesek, Anal. Chem. 61(18), 2067–2075 (1989).
- J.J. Pesek, and M.T. Matyska, J. Sep. Sci. 32(23–24), 3999–4011 (2009).
- J.J. Pesek, M.T. Matyska, and X. Pan, J. Chromatogr. A 992(1), 57–65, (2003).
- Z. Pan, M. Liu, C. Zheng, D. Gao, and W. Huang, Chinese J. Chem. 35 (8), 1227–123 (2017).
- H. Bai, Ind. Eng. Chem. Res. 51(50), 16457–16466 (2012).
- Y. Nakajima, and S. Shimada, RSC Adv. 5(26), 20603–20616 (2015).
- J. Soukup, and P. Jandera, J. Chromatogr. A 1245, 98–108 (2012).
- J. Soukup, and P. Jandera, J. Chromatogr. A 1228, 125–134 (2012).
- M. Holcapek, and W.C. Byrdwell, Handbook of Advanced Chromatography/Mass Spectrometry Techniques (Academic Press, London, United Kingdom, 2017), pp. 204.
- M. F. Wahab, D. C. Patel, R. M. Wimalasinghe, and D. W. Armstrong, Anal. Chem. 89(16), 8177–8191 (2017).
- J.M. Godinho, A.E. Reising, U. Tallarek, and J.W. Jorgenson, J. Chromatogr. A 1462, 165-9, (2016).
- J.P. Grinias, and R.T. Kennedy, Trends Analyt. Chem. 81, 110–117 (2016).
- D. Collins, E. Nesterenko, D. Connolly, M. Vasquez, M. Macka, D. Brabazon, and B. Paull, Anal. Chem. 83(11), 4307–13 (2011).
- A. Soliven, G.R. Dennis, E.F. Hilder, R.A. Shalliker, and P.G. Stevenson, Chromatographia 77(9), 663–671 (2014).
- T. Hara, G. Desmet, G.V. Baron, H. Minakuchi, and S. Eeltink, J. Chromatogr. A 1442, 42-52 (2016).
- S. Alharthi, and Z. El Rassi, Molecules 25(6), 1323 (2020).
- T.B. Kirchner, N.A. Hatab, N.V. Lavrik, and M.J. Sepaniak, Anal. Chem. 85(24), 11802–11808 (2013).
- S. Futagami, T. Hara, H. Ottevaere, G.V. Baron, G. Desmet, and W. De Malsche, J. Chromatogr. A 1523, 234–241 (2017).
- D.S. Bell, LCGC North Am. 36(6), 352–354 (2018).
- D. Neue, HPLC Columns: Theory, Technology, and Practice (John Wiley & Sons, Hoboken, New Jersey, 1997), pp. 119.

David S. Bell is a director of Research and Development at Restek. He also serves on the Editorial Advisory Board for LCGC and is the Editor for “Column Watch.” Over the past 20 years, he has worked directly in the chromatography industry, focusing his efforts on the design, development, and application of chromatographic stationary phases to advance gas chromatography, liquid chromatography, and related hyphenated techniques. His main objectives have been to create and promote novel separation technologies and to conduct research on molecular interactions that contribute to retention and selectivity in an array of chromatographic processes. His research results have been presented in symposia worldwide, and have resulted in numerous peer-reviewed journal and trade magazine articles. Direct correspondence to: LCGCedit@mmhgroup.com

Ahren I. Green is a ScientistII in the LC-R&D group at Restek Corporation, where he splits his time between synthetic work related to stationary phase construction and fine chemical synthesis for reference standard production.

Diego A. Lopez is a Scientist II in the LC-R&D group at Restek Corporation, where his role is to research, develop, and present on novel separation technologies, including new functionalization strategies for chromatography, and direct-to-MS applications.

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.








