Upgrading Gas Chromatography
This month's "GC Connections" explores the issues that can arise when deciding to upgrade or replace GC technologies.
Technology Advances
Technologies for laboratory analysis advance continuously, just as computer technologies or transportation technologies do. Small advances tend to occur fairly often while major new technologies appear less frequently. As new capabilities become available, laboratories must decide whether to acquire them or to defer and continue to use what they already have. Such decisions are reached by considering the roles and requirements of the laboratory, the short- and long-term costs of the new technologies, new skills that lab workers may have to acquire and the relative benefits and drawbacks of all of the changes.
Reasons and justifications for technology upgrades depend on laboratories' current and future needs. While the benefits of new capabilities are easy to describe, what may not be so evident are the collateral requirements of implementing new technologies in the lab. For example, switching to hydrogen carrier gas generation eliminates the costs of carrier gas cylinders and can yield faster speeds of analysis if hydrogen is not already in use, but the change also invokes some new safety requirements and procedures. This instalment of GC Connections discusses two related GC technologies and their impact on laboratory equipment and procedures.
Generate Your Own Gases
Installing carrier and flame gas generators is relatively easy, although there are some special considerations for hydrogen. The benefits of zero future gas cylinder costs plus no cylinder transport or demurrage charges yield an attractive return on investment (ROI), especially given the current high cost of carrier-grade helium. Zero-grade air generators are effective as well. The cost of detector-quality cylinder air is much lower than carrier-grade helium, but its "burn rate" is much higher at over 400 mL/min compared to a range of 50–250 mL/min for carrier gas with a split inlet system. Carrier gas consumption can be reduced by up to 80–90% if a gas-saver pressure-control mode turns off split flow while the inlet is not actively in use. There is no corresponding saver mode for FID air — a flame detector needs to stay lit and stable as long as there are pending analyses. Result — much more FID air is used in the average lab than carrier gas.
Gas generators have limited flow and pressure ranges that cannot be exceeded. It's a good idea to acquire gas generators that exceed current flow requirements by 25–50% to allow for future expansion. Also, installing gas generators will create a new requirement for regular generator maintenance, although arguably this is less effort than it takes to haul cylinders in and out of the lab.
Hydrogen: Generation of hydrogen for carrier and fuel gas invokes some additional concerns. For the majority of GC applications, hydrogen carrier gas can be substituted for helium — the exceptions are for certain fixed-gas separations as well as for some detectors, such as helium ionization (HID) and electron capture (ECD), in which helium actively participates in the detection chemistry. Even in these cases it is sometimes possible to apply helium as the make-up gas while using hydrogen as carrier gas, which will at least reduce helium consumption. As an alternative, most ECDs will work with a 5% methane in argon make-up gas mixture, although sensitivities and relative responses will change compared to helium. Mass spectrometric detectors are generally compatible with hydrogen carrier: some reduced pumping efficiency as well as lower background ionization levels can be expected. Also, some extra attention to proper detector venting is called for when shutting down, to avoid hydrogen accumulation inside the detector's vacuum chamber. Mass spectrometric detector manufacturers can provide detailed information about their specific products. For standard GC separations with FID, hydrogen carrier is an attractive choice because the same hydrogen source can also be used for the FID fuel gas. See reference 1 for some additional frequently asked questions about hydrogen carrier gas.
Switching to generated hydrogen carrier gas is a two-step process. First, if not already using hydrogen, install a tank of high-purity hydrogen — or use the existing FID hydrogen tank if it's pure enough — and validate performance with the new carrier. The column pressure settings will be different. Lower inlet pressures are required for the same average carrier gas linear velocities, while the optimum velocity for hydrogen is 10–20% higher than for helium. A flame ionization detector requires a constant flow of hydrogen fuel, which means electronic pneumatics will be needed to maintain flow when the column is temperature programmed. Run the carrier pneumatics in constant-flow mode if possible and establish a constant total FID hydrogen flow. Once the new carrier gas and detector settings have been validated, then consider switching from cylinders to a gas generator. From a cost point of view it may be easier to justify a new hydrogen generator if several GCs can be converted to hydrogen carrier at once.
Beyond considerations for method parameters, using hydrogen carrier gas will invoke some concerns for the potential burning or explosion hazards. A cylinder of flammable gas represents three distinct hazards. First, the very high pressure in any gas cylinder is a physical endangerment to personnel if not well understood and handled correctly. Second, the cylinder is very heavy and may present a lifting or falling hazard. Third, hydrogen is flammable and becomes explosive when mixed with air at concentrations between the lower and upper explosive limits of 4–74% by volume. A fully pressurized A-size cylinder at 2600 psig (18 kPa) contains nearly 8 m3 of gas when expanded to room pressure. In a small 20 × 30 ×10 foot (6 × 9 × 3 m) lab the lower explosive limit (LEL) of hydrogen could be reached if the entire contents of a cylinder were released at once. However, this extreme occurrence is very unlikely to take place by accident. Using a hydrogen generator to produce carrier and fuel gas relieves concerns for the release of a tankful of hydrogen — the generators store only a small amount of hydrogen at any time. Small amounts of hydrogen may be released into the lab from split vents or during column installation. For peace of mind, it may be a good idea to install a hydrogen sensor near the ceiling of the lab. I have one such sensor in my lab that gets tested — loudly — once in a while when I change the carrier gas to hydrogen and purge the carrier gas lines. But, I experiment with different carrier gases much more often than would a production lab.
Modern GCs include some safety features that address hydrogen concerns as well. Any lab that is considering hydrogen carrier is strongly urged to use an instrument with an electronic pressure control system that limits the flow of hydrogen carrier gas and detects and shuts off the flow under leakage or out-of-bounds conditions. Today's GCs feature explosion-safe ovens that, upon the extremely rare occasion of hydrogen accumulation and ignition, will contain the overpressure safely inside the oven. Hydrogen leak detector accessories are available for GC ovens, as well.
Increase Speed of Analysis with High Speed Gas Chromatography
Going to faster speeds of analysis with GC doesn't always require a new or upgraded instrument, but not all existing instruments are suitable. It all depends on how fast a separation is required. A modest increase of two to four times shorter retentions using conventional capillary columns with inner diameters of 200 μm or greater can be achieved quite reasonably on a wide range of conventional lab instruments. Very high speeds can achieve separations that previously needed 10 minutes or longer in less than one minute, but such a feat requires significant equipment upgrades or complete replacements. Pushing peaks from a column at high speeds places demands on autosamplers, inlets, detectors and data-handling systems that existing equipment may not be able to handle adequately. Newer GCs incorporate high-speed autosampler injection modes, appropriate inlet designs and fast data acquisition speeds that encompass the requirements for high speed operation up to a point. Specialized micro GC systems or dedicated rapid column heating accessories are required to go even further.
Making the decision to go to higher speeds is just the start of what can be an extended method development and validation exercise. A high-speed capable instrument is a platform on which to deploy a suitable column and method. It may be able to inject and record very narrow peaks while ramping up the column temperature at impressive rates, but without the necessary separation method it will not deliver the desired results.
Generally, faster linear velocities and higher temperatures or temperature programme rates, as well as reduced column lengths and inner diameters, will achieve a given separation in less time. This is attractive when considering whether to purchase additional GCs for the lab, and when faced with an increasing sample load. Alternatives such as adding a temporary work shift or contracting to an outside lab may be good intermediate solutions but in the longer term, increasing the sample throughput capacity of the lab is going to be more cost effective. But, the route to achieving higher analysis speeds can be difficult to traverse.

Certainly, hydrogen carrier gas is effective for faster GC separations because it is less viscous than helium and so produces higher linear velocities at the same pressures. The higher "speed limit" for hydrogen becomes important when pushing longer and narrower inner-diameter columns to yield faster separations — speeds of analysis are faster as inlet pressures approach their maximum values. Shifting to higher analysis speeds isn't a simple matter of cranking up the inlet pressure, however. A number of other parameters and considerations come into play in the GC fast lane.
First and foremost, the peaks of interest must be sufficiently resolved under a new set of conditions. If the separation is isothermal and if there is extra resolution to start with, then achieving higher speeds is relatively simple. Even when increasing pressures to operate well above optimum velocities, in such situations enough room between the peaks is available to produce adequate resolution at the higher speeds. All of the peaks move in proportion to an increase in velocity; their separations (a-factors) do not change.
Another approach to high speed separations involves going to smaller column inner diameters. Reducing the column i.d. while still operating at close to the optimum velocity will produce more theoretical plates, correspondingly narrower peaks and it will increase the resolution. Then the chromatographer can choose to crank up the inlet pressure and increase linear velocities away from the optimum, sacrificing the increased resolution of the narrower i.d. column for more speed.
Shifting peaks: Things get more complex with temperature-programmed separations, or when increasing isothermal temperatures. The elution times of all of the peaks decrease while the relative positions of dissimilar peaks shift as the temperature programme rate increases, which will also happen when increasing the temperature of an isothermal separation. This behaviour stems from the different ways in which solutes interact chemically with the stationary phase as temperatures change. In a temperature programmed run, modifying the pressure settings, or even changing from constant pressure to constant flow mode will also change relative peak positions. In this case, solutes experience slightly different temperatures as they move through the column more rapidly while the oven temperature programme profile is unchanged.
Reducing the column inner diameter is another approach to higher speeds, as already mentioned. However, with temperature programming there exists the possibility that peaks will shift in relation to each other if the column phase ratio (b) is not maintained while reducing the inner diameter. For example, a 530 μm i.d. column with a 1 μm stationary phase film has a phase ratio of
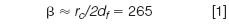
In a 250 μm i.d. column, with a potential of about a 1.4× higher theoretical plate count, the corresponding available film thickness for the same approximate phase ratio would be 0.5 μm. If the phase ratio is not kept about the same when changing column i.d., then dissimilar peaks in a temperature-programmed separation will again experience relative retention shifts.
This sensitivity of the separation of dissimilar peaks to changing column dimensions, temperatures and pressure conditions makes it necessary to optimize and validate any significant changes in an effort to achieve higher speeds. Simulation software such as Agilent's Method Translation software2 can significantly reduce the time and number of experiments required to reach a desired combination of speed and resolution from which to start formal method validation.
Achieving satisfactory results with high speed gas chromatography, then, requires both the right kind of host equipment and a suitably developed and validated separation. Modest speed increases are often possible with existing equipment, but truly high speed separations will require a commitment to obtaining the equipment and developing the methods. Crossing the bridge from conventional to high speeds may invoke more work than expected, but high speed separations can be well worth the extra effort when laboratory requirements demand them.
The Cascade Effect
In both cases presented here — advancing laboratory operations by switching to gas generation or by transitioning to higher speed analyses — the decision to improve laboratory capabilities will produce a successful outcome only if laboratory personnel fully consider the implications of the changes and perform the necessary predeployment tasks to ensure that the desired goals can be achieved. Changing to hydrogen carrier gas requires careful planning and consideration for both the potential added hazard as well as appropriate GC method changes. Upgrading to high-speed GC is a great idea, but performance requirements may necessitate the purchase of additional or replacement equipment. In any case, upgrading laboratory technologies is neither simple nor easy, but the results may be well worth the effort.
"GC Connections" editor John V. Hinshaw is a senior research scientist at Serveron Corp., Hillsboro, Oregon, USA and a member of the Editorial Advisory Board for LCGC Europe. Direct correspondence about this column should be directed to Alasdair Matheson via e-mail: amatheson@advanstar.com
For an ongoing discussion of GC issues with John Hinshaw and other chromatographers, visit the Chromatography Forum discussion group at www.chromforum.org
References
1. J.V. Hinshaw, LCGC Europe, 22(1), 32–37 (2009).
2. Available for download with no cost at http://www.chem.agilent.com/cag/servsup/usersoft/files/GCTS.htm
Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.














