A Universal Reversed‑Phase HPLC Method for Pharmaceutical Analysis
LCGC Europe
A universal generic high performance liquid chromatography (HPLC) or ultrahigh-pressure liquid chromatography (UHPLC) method with a primary modern column that works well for most drug analyses in a few minutes would be an attractive idea for many laboratories. With advances in column technologies, this ideal scenario is becoming more realistic, as demonstrated in the proposed 2-min generic method shown here. In addition, rationales for the selection of column and operating conditions are discussed, together with ways to extend this generic method as a starting point for stability-indicating applications by simple adjustments of gradient time and range.
Michael W. Dong, Perspectives in Modern HPLC Editor
A universal generic high performance liquid chromatography (HPLC) or ultrahigh-pressure liquid chromatography (UHPLC) method with a primary modern column that works well for most drug analyses in a few minutes would be an attractive idea for many laboratories. With advances in column technologies, this ideal scenario is becoming more realistic, as demonstrated in the proposed 2-min generic method shown here. In addition, rationales for the selection of column and operating conditions are discussed, together with ways to extend this generic method as a starting point for stability-indicating applications by simple adjustments of gradient time and range.
A generic high performance liquid chromatography (HPLC) method that works well for most small organic molecules is not a new idea. Most pharmaceutical laboratories use ballistic-gradient HPLC–ultraviolet (UV)–mass spectrometry (MS) methods for high-throughput screening (HTS), in-process control (IPC), and drug metabolism pharmacokinetics (DMPK) applications with gradient times (tG) ranging from 1 to 10 min depending on resolution (Rs) requirements (1,2). For instance, a 2-min reversedâphase ultrahighâpressure liquid chromatography (UHPLC) screening method using both acidic and basic mobile phases was routinely used for many years to support high-throughput purification in drug discovery (3). But how about extending this generic HTS–IPC approach further for multicomponent analysis or even purity assays? In 2012, a 10-min HPLC–UV cleaning verification method developed for a specific new chemical entity (NCE) was reported to work well for many drug candidates in a smallâmolecule portfolio with limits of quantitation down to 0.03–0.05 µg/mL (4). In 2013, the use of generic broadâgradient methods for purity analysis of raw materials, starting materials, and some active pharmaceutical ingredients (APIs) was proposed as part of a threeâpronged template approach strategy for rapid HPLC method development in pharmaceutical development (5). In this instalment, further refinements of this generic gradient method approach using the latest column technologies are proposed and exemplified in a 2-min HPLC assay for multiple NCEs. With the same column and mobile phase, higher peak capacities (Pc ≈ 200) and resolution can be obtained by increasing tG and gradient range–segments. Explanations for the selection of appropriate columns and operating conditions to maximize flexibility and compatibility with quality control (QC) applications are discussed together with ways this generic methodology can be extended for stability-indicating applications of more-complex drug molecules with simple adjustments of gradient conditions.
A Proposed Universal HPLC Method for Multiple NCEs
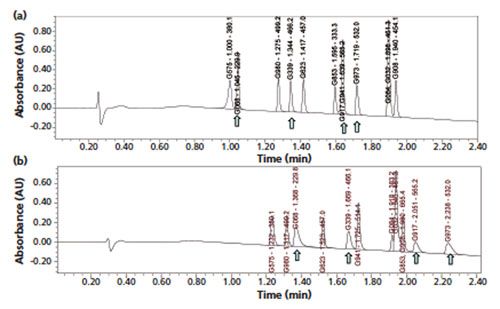
A 2-min generic gradient method amenable to the assays of multiple NCEs was proposed (6) and an example chromatogram on the separation of a test mixture of 12 NCEs is shown in Figure 1(a). These NCEs were randomly selected and represented drug candidates with optimized drug-like properties and binding constants to different disease targets with diversified pKa and log P values. The selection of the appropriate column, mobile phase, and operating conditions is discussed below. Note that the selection process and the final method parameters in this case study represented the end result, which balanced many analytical trade-offs (for example, analysis time, operating pressure, peak capacity, ease of use and ease of method transfers, and so forth) using available columns during laboratory evaluations. This example is used to illustrate the performance and usefulness of this generic gradient approach with modern columns under optimized operating conditions. It is expected that other similar choices on column and operating conditions may yield comparable and equally acceptable results.
Figure 1: Chromatograms of multiple NCEs obtained using the proposed universal HPLC method (a) with a 2.7-µm SPP C18 column and (b) with a 1.7-µm C18 column. Column (a): 50 mm × 3.0 mm, 2.7-µm Waters Cortecs C18+; column (b): 50 mm × 3.0 mm, 1.7-µm Waters BEH C18; mobile-phase A: 0.05% formic acid; mobile-phase B: acetonitrile; gradient: 5–60% B in 2 min, 60–95% B in 0.5 min, 95–5% B in 0.1 min; flow rate: 1.0 mL/min; temperature: 40 °C; pressure: 3300 psi; detection: UV absorbance at 220 nm and MS (ESI+); system: Waters Acquity UHPLC equipped with a 425-µL peptide mapping mixer with diode-array and MS detectors; dwell volume: ~0.5 mL; sample: 1 µL of test mix of 12 NCEs at 50–100 ng/mL. Peaks are designated by code name, retention time (min), and M+1 parent ion. (Figure 1[a] is adapted with permission from reference 6.)
The Column
In this case study, the column selected (support type, bonded phase, particle size, column length, and inner diameter) is based on chromatography principles (1,2), scientific literature, laboratory evaluations, considerations for QC applications, and compatibility with both HPLC and UHPLC equipment.
The selection of a superficially porous particles (SPP) column such as the Waters Cortecs column was well justified because of the significantly higher efficiency performance
(20–40%) versus those of their totally porous counterparts of the same particle diameter (7,8). The Cortecs C18+ bonded phase material was selected based on the better tailing factors produced for basic analytes (9,10). This C18+ phase has a positively charged surface, which yields superior peak shape performance (less tailing factors) for highly basic NCEs (with multiple positive charges under acidic pH, which are labelled with arrows in the chromatograms) when used with a low ionic strength mobile phase (for example, 0.05% formic acid) as shown in Figures 1(a) and 1(b) (11). Other column choices are discussed next.
A Cortecs C18+ column packed with sub-3-µm particles (dp = 2.7 µm) was selected as the primary column to provide better compatibility with both HPLC and UHPLC equipment for easier method transfers in global manufacturing situations (2). The Cortecs 1.6âµm SPP column was not chosen because the pressure drop is threefold higher (1), even though it can deliver higher efficiency and resolution. The 1.6-µm column in a narrow-bore format (2.1-mm i.d) would be the better choice for HTS applications using exclusively UHPLC equipment at high linear velocities (3).
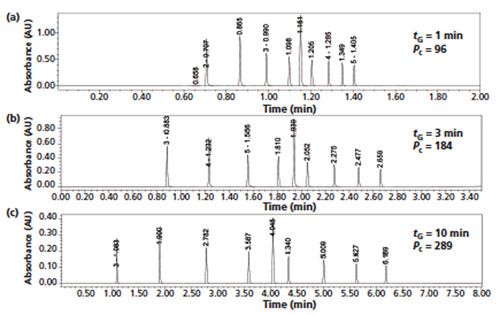
A short column length of 50 mm was selected for faster analysis. A short column allows excellent method flexibility for fast analysis and the ability of improving Pc by increasing tG (12,13). Figure 2 shows comparative chromatograms illustrating the increase of Pc from 100 to 300 by increasing tG from 1 to 10 min. This simple approach of increasing tG in conjunction with a narrower gradient range is an effective means to customize a generic method for stabilityâindicating assays, as demonstrated in the next section. The 3.0-mm i.d. format (rather than the more common UHPLC column format of 2.1 mm i.d.) was selected to yield better compatibility with HPLC equipment having higher system dispersion (1). The normal flow rates and injection volumes used for 3.0-mm i.d. columns are closer to those for standard 4.6âmm i.d. columns compared to those for 2.1âmm i.d. columns.
Figure 2: Comparative gradient UHPLC chromatograms obtained with gradient times of (a) 1 min, (b) 3 min, and (c) 10 min showing the effect on peak capacity. Column: 50 mm × 2.1 mm, 1.6-µm Cortecs C18+; mobile-phase A: 0.05% formic acid; mobile-phase B: acetonitrile; gradient: 30–100% B; flow rate: 1 mL/min; temperature: 40 °C; pressure: 12,000 psi; detection: UV at 254 nm; sample: a test mixture of nine alkylphenones. Peak capacities were measured by dividing the gradient time by the average measured base peak widths.
The Mobile Phase
A simple mobile-phase A of 0.05% formic acid was initially selected to permit easy mobile phase preparation in addition to its ability to support excellent mass spectrometry (MS) ionization efficiency (14). An alternate mobile-phase A of 20 mM ammonium formate with a higher ionic strength buffered at pH 3.7 would likely yield better peak shapes for most NCEs on a wider selection of bondedâphase columns (10,11). A buffered mobile phase may also be beneficial for critical separations that require tighter control of the mobile phase pH (14). Nevertheless, the simple formic acidâonly mobile phase worked well, yielding good peak shapes for all NCEs tested with the recommended columns in this case study. An example chromatogram on the separation of the same 12-NCE test mixture by the primary column using this formate buffer is shown in Figure 3(a). Example chromatograms showing results for the 12-NCE test mixture using the primary Cortecs C18+ column for mobile-phase A with formic acid and ammonium formate are shown in Figures 1(a) and 3(a), respectively. Note that the chromatogram in Figure 3(a) shows analytes that have higher retention and different selectivity in comparison to those in Figure 1(a) because of higher mobile phase pH (pH 3.7 versus ~3.0) and ionic strength.
Figure 3: Chromatograms of the 12 NCE test mix obtained (a) using the same conditions as in Figure 1(a) but with 20 mM ammonium formate pH 3.7 as mobile‑phase A and (b) after adjusting the gradient conditions. Conditions in (b): column: 50 mm × 2.1 mm, 2.7-µm Waters Cortecs C18+; mobile-phase A: 0.05% formic acid; mobile-phase B: acetonitrile; gradient: 5–40% B in 5 min, 40–95% B in 0.5 min; flow rate: 1.0 mL/min; temperature: 40 °C; pressure: 5300 psi; detection: UV absorbance at 254 nm and MS. Pressure in (a): 3700 psi. (Figure 3[b] is adapted with permission from reference 6.)
Acetonitrile, used as mobile-phase B, provides higher peak capacities and lower pressure drop than methanol because of acetonitrile’s lower viscosity (1). Acetonitrile with 0.03% formic acid (v/v) should be used with 0.05% formic acid (v/v) (balanced absorbance mobileâphases A and B) to provide a flatter baseline with UV detection at low wavelengths (such as <230 nm).
Gradient Conditions
A two-segment gradient programme of 5–60% B and 60–95% B (rather than a single broad gradient of 5–95% acetonitrile) has been demonstrated to be more appropriate for most NCEs (4),
which tend to be less hydrophobic and are typically designed according to the Lipinski’s “rule
of five” in drug discovery (15). A
quick purging gradient is
customarily needed to elute hydrophobic impurities such as dimers. A flow rate of 1.0 mL/min is optimum for this preferred column (2.7 µm, 50 mm × 3.0 mm) (14) and a column temperature of 30–40 °C is also quite standard. An initial operating pressure of 3300 psi
is observed for this universal method, which renders it compatible with both HPLC and UHPLC equipment. UHPLC systems are preferable from the standpoints
of lower dwell volume (shorter gradient delay times) and system dispersion (less extracolumn bandbroadening), leading to a faster sample turnaround time, taller and narrower peaks, and higher resolution (1,9).
Detection
A UV detection wavelength of 220 or 254 nm was typically used for this method and could be changed readily to match the maximum absorption wavelengths (λmax) of the NCEs to maximize UV detection sensitivity (1,2). A typical MS scanning range of 150–1000 amu using electrospray ionization in the positive mode (ESI+) was found to work well for most small-molecule NCEs.
Resolving all NCEs in the Test Mixture Using a Modified Generic Method
As demonstrated by the chromatogram in Figure 1(a), the proposed universal gradient method with a tG of 2 min is capable of separating 10 peaks with excellent peak shapes in a sample test mixture of 12 NCEs. This fast, 2-min method may be adequate for potency assays for multiple NCEs or as a standard generic HPLC–UV–MS method for cleaning verification assay (4). As mentioned earlier, higher peak capacities are easily obtainable by increasing tG and by adjusting the gradient range to separate any critical pairs in the sample. As was shown in the chromatogram in Figure 3(b), all 12 NCEs could be near-baseline resolved with the same mobile phase and bonded phase used in Figure 1(a) by simple adjustments of a narrower gradient range and a longer tG (5% to 40% B in 5 min) (5).
Can the Proposed Generic Method Be Modified for Stability-Indicating Assays?
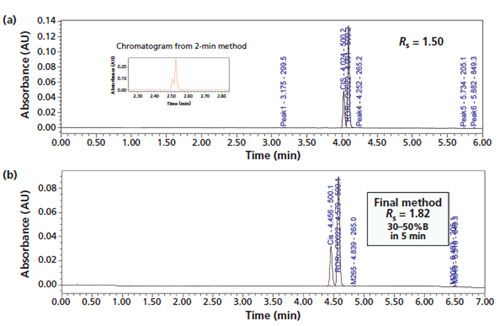
This generic method appeared to serve well as a starting point for stabilityâindicating assays of many NCEs. To illustrate this approach, a challenging stability-indicating method was developed for an NCE with a cis-isomer (both an impurity and a degradant). The method development process using a sequence of method adjustments is shown in Figure 4. First, a particular NCE sample spiked with the cisâisomer was injected using the 2-min generic method shown in Figure 1(a) (5–60% B in 2 min) yielding a partial separation (Rs = 0.8) of the two isomers shown in the inset of Figure 4(a). Next, a narrower gradient range of 20–60% B in 5 min was used to yield the chromatogram in Figure 4(a) showing Rs = 1.50 for the isomers. The chromatogram using final separation conditions (30–50% B in 5 min) is shown in Figure 4(b), producing Rs = 1.82 for the isomers. This approach of maximizing the resolution around the main peak by keeping its elution near the end of a shallow gradient segment has been described elsewhere (5). A short first segment of 5–30% B in 0.5 min was added to retain any potential earlyâeluting impurities or degradation products. The entire method development process for this 6-min, three-segment gradient method took only 1 h in this case without the aid from any computer simulation software (1). Next, this 6-min method was successfully used to analyze a series of forced degradation samples to verify method specificity. This modified 6-min method separated all the impurities and degradation products (identified by their M+1 base peaks) and was subsequently used in a release testing of a pivotal API lot for toxicology evaluation.
Figure 4: A case study on development of a stability-indicating method for an NCE with a cis isomer using the generic method approach: (a) Chromatogram of the NCE sample spiked with the cis isomer with a revised gradient programme of 20–60% B in 5 min. The chromatogram in the inset shows the partial resolution (Rs = 0.8) of the two isomers using the 2-min generic method in Figure 1(a). (b) Chromatogram of the final stability-indicating method with a total method development time of only 1 h. Column: 50 mm × 3.0 mm, 2.7-µm Waters Cortecs C18+; mobile-phase A: 0.05% formic acid; mobile-phase B: acetonitrile; gradient: 5–30% B in 0.5 min, 30–50% B in 5 min, 50–95% in 0.5 min; flow rate: 1.0 mL/min; temperature: 35 °C; pressure: 3700 psi; detection: UV absorbance at 254 nm and MS 150–999 amu ESI+.
The results from the forced degradation study (Table 1) indicated that this NCE is relatively stable in solid form in the presence of heat and moisture, but it can degrade quickly in solution (50% acetonitrile in water), particularly at acidic or basic pHs.
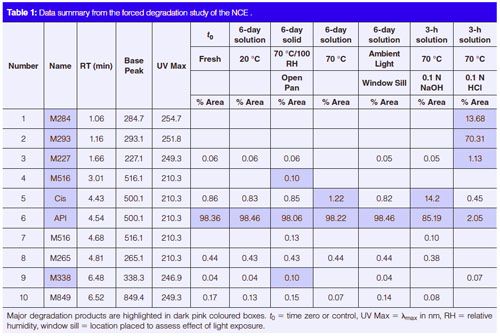
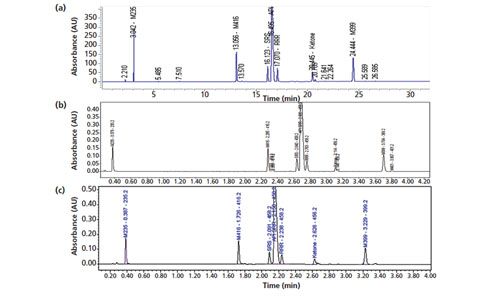
This method development approach was also applied to a more complex drug molecule with three chiral centres. The performance of the existing 42-min regulatory HPLC method in the separation of a retention marker solution of the API spiked with expected impurities and degradation products (including the two expected diastereomers) is shown in Figure 5(a) and has been described in an earlier publication (16). The performance of a 5-min, two-segment gradient method developed using the generic method approach is shown in Figure 5(b). The total method development (adjustment) process took about 1 h using the primary column with the same mobile phase A in the regulatory HPLC method (20 mM ammonium formate buffer at pH 3.7). The exact same method conditions were found to work well for a similar SPP column (50 mm × 3.0 mm, 2.7âµm Agilent Poroshell HPH-C18) as shown in Figure 5(c).
Figure 5: Chromatograms obtained using (a) the existing 42-min regulatory HPLC method for a complex NCE molecule with three chiral centres, (b) the 6-min, two‑gradient segment method with the generic method approach using a Waters Cortecs C18+ column, and (c) the 6-min, two-gradient segment method with the generic method approach using an Agilent Poroshell HPH-C18 column. Conditions in (a): column: 150 mm × 4.6 mm, 3-µm ACE C18; mobile-phase A: 20 mM ammonium formate, pH 3.7; mobile-phase B: 0.05% formic acid in acetonitrile; gradient: 5–15% B in 5 min, 15–40% B in 25 min, 40–90% B in 10 min, 90% B in 3 min, 90–5% B in 0.1 min; flow rate: 1.0 mL/min; temperature: 30 °C; pressure: 3500 psi; detection: UV absorbance at 280 nm; sample: 10 µL of ~0.5-mg/mL API spiked with expected impurities. Conditions in (b): column: 50 mm × 3.0 mm, 2.7-µm Waters Cortecs C18+; gradient: 5–40% B in 4 min, 40–95% B in 1 min; temperature: 35 °C; sample: 2 µL of the retention marker solution; other conditions were the same as in (a). Conditions in (c): 50 mm × 3.0 mm, 2.7-µm Agilent Poroshell HPH-C18; pressure: 4000 psi; other conditions were the same as in (b). (The chromatogram in Figure 5[a] was adapted with permission from reference 16.)
An Alternate Column
Although the Cortecs C18+ 2.7 µm column worked well for the proposed 2-min generic method with acidic mobile phases, other subâ3âµm SPP columns from different manufacturers such as those from Advanced Chromatography Technologies (ACT), Agilent, Advanced Materials Technology (AMT), Supelco, Thermo Scientific, and Phenomenex may also be considered (7), although one may observe more peak tailing for very basic analytes when using lowâionicâstrength mobile phases in some of the columns. Potential shortcomings of the Cortecs C18+ column are more peak tailing with acidic analytes and its incompatibility with high-pH mobile phases (1,17), which have the advantage of improving retention and peak shape for basic analytes, and are useful for the development of MS-compatible ICHâcompliant purity methods for water-soluble basic drugs (17).
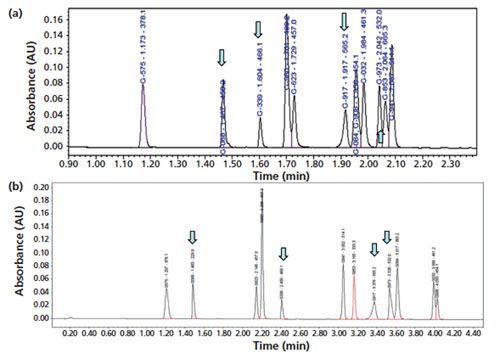
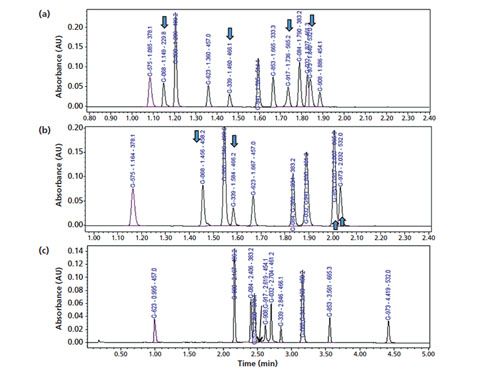
Potential alternative columns for high-pH mobile phase applications are the Waters CSH C18 (nonâSPP charged surface hybrid) and Agilent Poroshell HPH-C18 (an SPP for high-pH applications) columns (18). Chromatograms demonstrating the performance of the Poroshell HPHâC18 column using a mobileâphase A consisting of 0.05% formic acid, formate buffer, and 0.05% ammonia are shown in Figures 6(a–c). This column was also found to yield excellent peak shapes for very basic analytes with 0.05% formic acid mobile phase (Figure 6[a]) even though the bonded phase may not have a charged surface. Note that one serious issue for the use of highâpH mobile phases is the increased potential for analyte degradation as shown in Figure 6(c) (with one NCE [G-917] suffering severe degradation during analysis).
Figure 6: Chromatograms of the separation of the 12-NCE test mix obtained using the alternate column (50 mm × 3.0 mm, 2.7-µm Agilent Poroshell HPH-C18) and (a) the proposed generic method conditions with 0.05% formic acid mobile-phase A as in Figure 1(a), (b) 20 mM formate buffer as mobile-phase A, and (c) 0.05% ammonia as mobile-phase A and a gradient programme of 15–60% B in 5 min.
Strengths and Limitations of the Universal Gradient Method
The strengths of this modernized generic HPLC method are: its flexibility to provide good separations (Pc = 100–200); short run times (2–6 min); and good peak shapes for multiple NCEs. The generic method can be customized quickly for stability-indicating assays by simple adjustments of tG and gradient range–segment. One limitation of the recommended 50-mm columns is their relatively low column efficiency (N = 12,000). Longer versions (100–150 mm) of these columns (capable of higher Pc of 200–400) are therefore more appropriate for the final regulatory stability-indicating methods for moreâcomplex NCEs (19,20). Nevertheless, these short columns are excellent candidates for column and mobile phase screening studies and for purity assays of raw materials, starting materials, and less complex NCEs (6,12,20).
Summary and Conclusion
A 2-min universal HPLC–UV–MS method for pharmaceutical analysis using modern SPP columns under optimized operating conditions is proposed. The advantages of this generic method are fast analysis, reasonable peak capacity (~100), and excellent peak shapes for many NCEs. This method may function as a general assay method for multiple NCEs and a standard method for cleaning verification. It also appears to serve well as a starting point for the development of stabilityâindicating assays of many drug molecules using a multigradient segment approach with a longer gradient time.
Acknowledgements
The authors would like to thank Naijun Wu of Celgene, Xiaotong Zhang of Allergan, Jacob Fairchild and Pam Iraneta of Waters Corporation, Hernan Fuertes of Gilead Sciences, Tom Waeghe of MAC-MOD Analytical, and Davy Guillarme and Szabolcs Fekete of the University Geneva for their editorial inputs and suggestions.
References
- M.W. Dong, Modern HPLC for Practicing Scientists (Wiley, Hoboken, New Jersey, USA, 2006), chapters 2,3,7.
- B. Kassel, in HPLC for Pharmaceutical Scientists, Y. V. Kazakevich and R. LoBrutto, Eds. (Wiley, Hoboken, New Jersey, USA, 2007), chapter 11.
- M. Wong, B. Murphy, J. H. Pease, and M.W. Dong, LCGC Europe28(6), 343–352 (2015).
- M.W. Dong, E.X. Zhao, D.T. Yazzie, C. C. Gu, and J. D. Pellett, Amer. Pharm. Rev.15(6), 10–17 (2012).
- M.W. Dong, LCGC Europe 26(8), 455–461 (2013).
- M.W. Dong, LCGC Europe 29(6), 328–337 (2016).
- S. Fekete, D. Guillarme, and M.W. Dong, LCGC North Am.32(6), 420–433 (2014).
- S. Fekete, E. Oláh, and J. Fekete, J. Chromatogr. A1228, 57–71 (2012).
- D. Guillarme and M.W. Dong, Amer. Pharm. Rev. 16(4), 36–43 (2013).
- K.J. Fountain, H.B. Hewitson, P.C. Iraneta, and D. Morrison, “Practical Applications of CSH Technology,” Waters Corporation, Milford, Massachusetts, USA, 720003720EN, Sep 2010.
- D.V. McCalley, Anal. Chem. 78, 2532 (2006).
- M.W. Dong and K. Zhang, Trends in Anal. Chem. 63, 21–30 (2014).
- D. Guillarme, E. Grata, G. Glauser, J.-L. Wolfender, J.-L. Veuthey, and S. Rudaz, J. Chromatogr. A1216, 3232–3243 (2009).
- M.W. Dong, LCGC North Am.32(8), 552–557 (2014).
- C.A. Lipinski, F. Lombardo, B.W. Dominy, and P.J. Feeney, Advance Drug Delivery Reviews 23, 3–25 (1997).
- M. Dong, D. Guillarme, S. Fekete, R. Rangelova, J. Richards, D. Prudhomme, and N.P. Chetwyn, LCGC North Am.32(11), 868–76 (2014).
- M.W. Dong, G. Miller, and R. Paul, J. Chromatogr. A 987, 283–290 (2003).
- W.J. Long, A.E. Mack, X. Wang, and W.E. Barber, LCGC Europe29(s6), 31–38 (2015).
- P.W. Carr, X. Wang, and D.R. Stoll, Anal. Chem.81, 5342–5353 (2009).
- P.W. Carr, D.R. Stoll, and X. Wang, Anal. Chem.83, 1890–1900 (2011).
Michael W. Dong is a principal of MWD Consulting, which provides training and consulting services in HPLC and UHPLC, pharmaceutical analysis, and drug quality. He was formerly a Senior Scientist at Genentech, Research Fellow at Purdue Pharma, and Senior Staff Scientist at Applied Biosystems/Perkin-Elmer. He holds a PhD in Analytical Chemistry from City University of New York. He has more than 100 publications and a best-selling book in chromatography.
He is an editorial advisory board member of LCGC North America. Direct correspondence to: LCGCedit@ubm.com


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









