Unique Effects of Two Mobile Phase Alcohols on the RegisPack CLA-1 Chiral Stationary Phase (CSP)
Typical chiral stationary phase (CSP) screening paradigms utilize a single alcohol component as co-solvent in its mobile phase. Recent unexpected observations have demonstrated that mixed-alcohol mobile phases can enhance or even introduce peak resolution when none existed in a mono-alcohol system.
Typical chiral stationary phase (CSP) screening paradigms utilize a single alcohol component as co-solvent in its mobile phase. Recent unexpected observations have demonstrated that mixed-alcohol mobile phases can enhance or even introduce peak resolution when none existed in a mono-alcohol system.
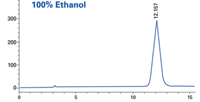
Figure 1: Sample: proprietary; column: RegisPack® CLA-1 25 cm à 4.6 mm; mobile phase: 100% ethanol; flow rate: 1.0 mL/min.
The standard protocol for screening with alcohol co-solvents such as ethanol, methanol, and isopropanol is to execute the screen with just a single alcohol. In this particular example, ethanol and methanol were used individually to perform the initial screen. When either alcohol alone was used, there was no resolution suitable for a preparatory scale separation. Select mixtures of the two alcohols showed an unexpected separation of the enantiomers of the racemate sample (compound structure is confidential).
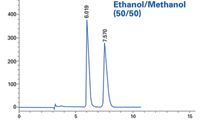
Figure 2: Sample: proprietary; column: RegisPack® CLA-1 25 cm à 4.6 mm; mobile phase: ethanol/methanol (50/50); flow rate: 1.0 mL/min.
Unlike the unchlorinated RegisPack® amylose-based CSP, this study used the newer chlorinated (tris-(5-chloro-2-methylphenyl) carbamoyl amylose)-based RegisPack® CLA-1. The column dimensions were 25 cm × 4.6 mm using a 5 µm particle size.
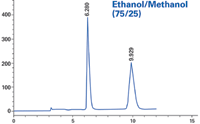
Figure 3: Sample: proprietary; column: RegisPack® CLA-1 25 cm à 4.6 mm; mobile phase: ethanol/methanol (75/25); flow rate: 1.0 mL/min.
Conclusion
Using neat alcohols will typically give acceptable peak resolution of a racemate, provided the CSP is appropriate. This particular compound, however, exhibited no peak resolution when using 100% of a single alcohol as co-solvent. Combinations of the two alcohols gave excellent baseline separations of the example compound. The solvent-CSP interaction, while not obvious, provides an interesting option when trying to develop conditions for difficult separations. Thus, when the two neat alcohols alone don't show promise, one should consider mixtures before ruling a screen unsuccessful.
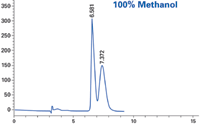
Figure 4: Sample: proprietary; column: RegisPack® CLA-1 25 cm à 4.6 mm; mobile phase: 100% methanol; flow rate: 1.0 mL/min.
Regis Technologies, Inc.
8210 Austin Ave, Morton Grove , IL 60053
tel. (847) 583-7661, fax (847) 967-1214
e-mail: teds@registech.com, Website: www.registech.com/chiral

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














