Semi-Quantitative Determination of Volatile Oligomers of Halogenated Compressor Oil in a Manufacturing Process Using a Person Portable GC–MS
During the manufacturing process of industrial gases, volatile oligomers of compressor fluids can enter the product stream. These impurities are typically within specification, but failure of a diaphragm or seal can spike the concentration in a fill batch and the industrial gas no longer meets product specifications. A screening method has been developed to extract, identify and quantitate chlorotrifluoroethylene-based impurities from the compressor fluid using a CUSTODION? solid phase microextraction (SPME) syringe and a TRIDION?-9 person-portable gas chromatograph–toroidal ion trap mass spectrometer (GC-TMS). The compounds were separated and detected rapidly in less than 5 min analysis time.
During the manufacturing process of industrial gases, volatile oligomers of compressor fluids can enter the product stream. These impurities are typically within specification, but failure of a diaphragm or seal can spike the concentration in a fill batch and the industrial gas no longer meets product specifications. A screening method has been developed to extract, identify and quantitate chlorotrifluoroethylene-based impurities from the compressor fluid using a CUSTODION™ solid phase microextraction (SPME) syringe and a TRIDION™ -9 person-portable gas chromatograph–toroidal ion trap mass spectrometer (GC-TMS). The compounds were separated and detected rapidly in less than 5 min analysis time.
In this screening method a CUSTODION SPME syringe offers a unique sampling capability where the syringe needle can be exposed to the gas containers via an appropriate interface. The SPME fiber concentrates trace amounts of the volatile components of compressor fluid. Using a TRIDION-9 GC–MS to analyze samples is fast and decision making can take place during the fill process or at least before the product container is sent to a customer. The SPME-GC–MS method can provide contaminant trace analysis at ppb level, which is typically below the lower limit of acceptance for impurities in industrial gases. This analytical method can save time and money by eliminating rework, and can assure the customer that the product meets the specification.
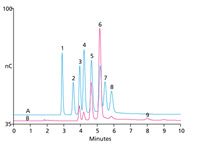
Figure 1: Total ion chromatogram of the volatile chlorotrifluoroethylene oligomers (sample 2).
Experimental Conditions
Bromopentafluorobenzene (BPFB) and dibromotetraflurobenzene (DBTFB) were spiked into a Tedlar™ bag at known vapor concentrations, and were used as reference standards. Liquid compressor oil samples were weighed into vials that simulated gas containers. The volatile oligomers were allowed to equilibrate between the liquid and vapor phases (air, at ambient pressure) for 24 h. Both the standards and sample analytes were extracted from the gas phase at ambient temperature for 2 min using a CUSTODION SPME syringe with a 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) fiber.
Following each sample extraction, the SPME syringe was inserted into the TRIDION-9 GC-TMS injection port where the target analytes were desorbed into a split-splitless injector (280 °C) coupled with a low thermal mass metal-clad capillary GC column (MXT-5, 5 m × 0.1 mm, 0.4 µm df). After an initial 10 s hold at 40 °C, the GC temperature was increased at 2 °C/s to 280 °C for a total run time of 2 min and 20 s. The capillary GC is coupled to a TMS detector having a mass range of 45–500 m/z.
Total ion chromatogram area of the samples and standards were used for quantitation. Semi-quantitative results are often applied for in-field measurements. The procedures for in-field calibration are designed to be simple with minimal preparation and generate a level of data quality that provides actionable results.
Results
Figure 1 shows the GC-TMS analysis of the volatile chlorotrifluoroethylene oligomers samples.
Conclusions
The CUSTODION SPME syringe and TRIDION-9 GC-TMS are uniquely suited for rapid quality assurance analysis of product gases for compressor oil oligomers and other organic impurities in the gas phase. This method can be performed at the filling location allowing decisions regarding product purity and filling process integrity be made in real-time. The ~5 min total analysis time makes it possible to collect additional samples within the timeframe of the batch fill.
Acknowledgments
Torion®, CUSTODION™ and TRIDION™ are trademarks of Torion Technologies Inc. The CUSTODION SPME Syringes are manufactured and sold under license from Supelco under US Patent 5,691,206, and/or any divisions, continuations, or revisions thereof.
Torion Technologies Inc.
796 East Utah Valley Drive, Suite 200, American Fork, UT 84003
tel. (801) 705-6600
Email: information@torion.com, Website:www.torion.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















