Ultrahigh-Pressure Size-Exclusion Separations of Engineering Plastics: Challenges and Opportunities
LCGC Europe
Ultrahigh-pressure size-exclusion chromatography (UHPSEC) can potentially offer a new direction and overcome some of the disadvantages (for example, limited resolution and long analysis time) associated with conventional SEC analysis. UHPSEC is based on using sub-3-µm silica-organic hybrid particles under elevated pressures (often above 400 bar) to improve the separation efficiency and increase analysis speed. In spite of the benefits promised by the chromatographic theory, there are some uncertainties that may limit the proliferation of UHPSEC in polymer analysis. As a result of possible issues associated with the use of a different stationary phase - including secondary interactions and polymer degradation - it is unclear whether UHPSEC will provide results comparable to those of traditional SEC methods. In this article, the advantages and challenges of UHPSEC for the analysis of different engineering thermoplastics are discussed, as well as a comparison of results obtained with UHPSEC and conventional SEC.
Photo Credit: Srebrina Yaneva/Getty Images

Ultrahigh-pressure size-exclusion chromatography (UHPSEC) can potentially offer a new direction and overcome some of the disadvantages (for example, limited resolution and long analysis time) associated with conventional SEC analysis. UHPSEC is based on using sub-3-µm silica-organic hybrid particles under elevated pressures (often above 400 bar) to improve the separation efficiency and increase analysis speed. In spite of the benefits promised by the chromatographic theory, there are some uncertainties that may limit the proliferation of UHPSEC in polymer analysis. As a result of possible issues associated with the use of a different stationary phase - including secondary interactions and polymer degradation - it is unclear whether UHPSEC will provide results comparable to those of traditional SEC methods. In this article, the advantages and challenges of UHPSEC for the analysis of different engineering thermoplastics are discussed, as well as a comparison of results obtained with UHPSEC and conventional SEC.
Size-exclusion chromatography (SEC) is a very common technique for polymer characterization. Though many developments have occurred in the liquid chromatography (LC) area in recent decades, they have hardly impacted on SEC. In most laboratories SEC is still conservatively performed using relatively long columns (or several columns connected in series) packed with 5–10 µm polymeric particles. The analysis time is usually long (15 min per column) and the efficiency is low. Because of the growing demand for efficient high-speed, high-throughput characterization in analytical laboratories (for example, in quality control environment), it is important to explore new approaches for polymer molecular-weight characterization. According to the chromatographic theory (1), smaller stationary phase particles provide lower chromatographic band broadening and therefore offer better separation efficiency compared to larger particles. In addition, columns packed with smaller particles can be operated at higher flow rates without severe loss of efficiency because the plate height does not increase at higher linear velocities as dramatically as in the case of larger particles. Therefore, stationary phases with smaller particles can be used to improve separation quality and speed. However, the use of such packings brings special requirements for the chromatographic instrumentation. In order to obtain the benefits in practice, the columns need to be operated at linear velocities that are sufficiently high (at or above the optimum of the van Deemter curve [1]). The backpressure generated by such columns at optimum flow rates is often higher than the pressure capabilities of conventional liquid chromatography (LC) equipment. Therefore, instruments with higher pressure limits are required. For that reason, the technique received the name ultrahigh-pressure liquid chromatography (UHPLC). In addition, the instruments need to possess low dead volumes in both the chromatograph and detector to preserve the obtained separation. Because of the advantages they offer and because of the commercial availability of UHPLC instrumentation, usage of small particles is a natural trend in separation science. Sub-2-µm particles in interaction LC have been used for more than a decade already, bringing significant improvements in analysis speed and efficiency to analytical chemists in many application fields. However, polymer characterization by ultrahigh-pressure size-exclusion chromatography (UHPSEC) is a less mature technique. Although several studies have demonstrated the high potential of such polymer separations (2,3), the practical applications of UHPSEC so far are limited. This was mainly because no commercial packing materials existed that could accommodate large pore sizes and large pore volume (requirements for SEC) in very small particles that would be sufficiently stable at ultrahigh pressures. Moreover, some concerns were raised by the chromatographic community questioning the feasibility and usefulness of UHPSEC (4,5). These were related to possible degradation of polymers at UHP conditions. Recently stationary phases for UHPSEC have become commercially available that have enabled application of this technique for the separation of different polymers and a detailed evaluation of its advantages and potential drawbacks. The following sections will address the challenges and benefits of UHPSEC.
Experimental
Narrow-disperse polystyrene standards were obtained from Agilent Technologies in the molecular weight range from 580 to 1,000,000 Da. HPLC-grade dichloromethane (amylene stabilized) and chloroform (ethanol stabilized) were obtained from Fisher Scientific. Hexafluoroisopropanol (HFIP) was obtained from Acros organics. Dibutylamine and polymer samples (polycarbonate, polyetherimide, polyphenylene ether, and polybutylene terephthalate) were obtained from an in-house supply. All samples and standards were dissolved in respective solvents at concentrations of 1 mg/mL for the testing. Toluene (Acros Organics) was applied as a flow marker at a concentration of 250 ppm. Samples and standards were filtered through 0.45 µm PTFE syringe filters from Fisher Scientific before analysis. The Waters APC system used for the UHPSEC separations was fitted with an isocratic ultrahigh pressure pump, sample and column manager, and a photo-diode array (PDA) detector. Detection was made at 254 nm. All UHPSEC columns used in this study were obtained from Waters (Acquity APC XT column family). Three UHPSEC columns with dimensions 150 × 4.6 mm and pore sizes of 450, 200, and 125 Å were used in series. For fast UHPSEC experiments two UHPSEC columns with dimensions of 75 × 4.6 mm and pore sizes of 450 and 125 Å were used. For oligomer separations a UHPSEC column with dimensions 150 × 4.6 mm and a pore size of 45 Å was applied. The particle size was 1.7 µm for the 45 Å column and 2.5 µm for columns with larger pores. The injection volume was 5 µL. The flow rate was 1 mL/min or 2 mL/min (for fast UHPSEC experiments). For conventional SEC an Agilent 1100 system was used with an isocratic pump, autosampler, column oven, and PDA detector set at 254 nm. For analysis of polybutylene terephtalate a PLgel Minimix HFIP Gel SEC column was applied; for the analysis of oligomers an OligoPore oligomeric SEC column with a pore size of 100 Å was used. For all other polymers a PLgel MiniMix C SEC column was applied. All SEC columns were obtained from Agilent Technologies and had dimensions of 250 × 4.6 mm and particle size of 5 µm. A flow rate of 0.3 mL/min was used. The injection volume was 10 µL. The data was reported in polystyrene units. All SEC columns were based on a polystyrene/divinylbenzene stationary phase.
Results and Discussion
Challenges in UHPSEC Analysis
Secondary Interactions in UHPSEC:
Commercially available UHPSEC columns have a different chemistry compared to the columns that are conventionally used for SEC. The most typical stationary phase for SEC is made of styrene-divinylbenzene material – a polymeric stationary phase that does not have ionic groups. This helps to minimize any secondary (other than pure exclusion) interactions of the analytes with the stationary phase. UHPSEC columns commercialized by Waters – currently the single manufacturer of such columns – contain silica-organic hybrid material. The packing consists of ethoxylated silica particles with ethylene bridges between them. Such ridged structures makes the material resistant to elevated pressures (above 400 bar) even while having large pores and large pore volumes incorporated into small sub-3-µm particles. However, the presence of silica and silanol groups increases the risk of ionic interactions and the adsorption of analyzed macromolecules onto the stationary phase. Therefore, in order to evaluate the performance of UHPSEC it is crucial to examine whether such interactions occur in practice and whether they significantly affect analytical results. To understand this, molecular weights obtained with UHPSEC were compared to those obtained by conventional legacy SEC for several types of plastics (polycarbonate, polyetherimide, polyphenylene ether, and polybutylene terephthalate). The polymers were selected to cover a wide range of properties: polarity, solubility, chemical structure, and end-group variety. These macromolecules exhibit very different chromatographic behaviour and require different conditions for SEC separations. Based on the results of this study the behaviour of other (similar) type of polymers in UHPSEC can be anticipated.
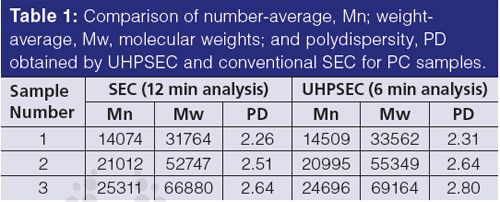
Bisphenol Acetone Polycarbonate (BPA PC):
BPA PC is a non-polar polymer that is well-described in the literature (6). It is soluble in non-polar solvents such as chloroform or dichloromethane and it can be analyzed by SEC in these solvents. Several PC samples with different molecular weights with UHPSEC in dichloromethane were studied and the results were compared with those obtained by conventional SEC (Table 1). The Mn values are very close and the differences are within experimental error (see also average values of five injections in Table 2). The Mw values are somewhat higher with UHPSEC and that results in higher polydispersity (PD) values. However, the absolute values are in a good agreement. UHPSEC columns based on a silica-organic hybrid technology do not have any active sites that may noticeably interact with PC chain or end-groups (typical end-groups are OH and phenol for example). Therefore, SEC and UHPSEC provided comparable data.
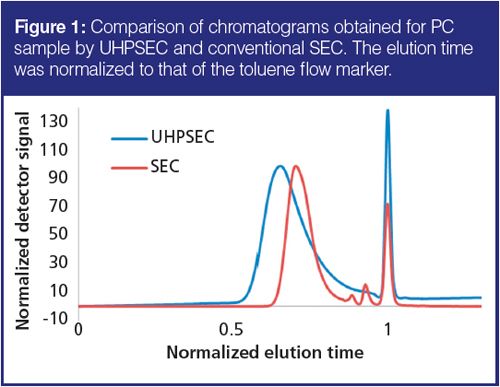
The chromatograms obtained by SEC and UHPSEC are shown in Figure 1. The elution time was normalized to that of a flow marker (toluene) to aid visual comparison. The actual analysis time was 6 min for UHPSEC and 12 min for SEC. Note that the shape of the UHPSEC chromatogram is different from that of the SEC chromatogram. UHPSEC shows higher tailing in the low-molecular-weight region. This can be explained by the higher selectivity of the UHPSEC columns in this region as a result of the application of three single-pore size columns rather than a linear column (as in the case of SEC).
Polyetherimide (PEI):
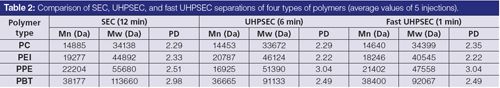
PEI is a nitrogen-containing polymer that makes it susceptible to ionic interactions and adsorption. It is also known to contain a variety of end-group functionalities (7) that will contribute to non-ideal SEC behaviour of this polymer (also in conventional SEC). Dichloromethane was used as a solvent and eluent for the PEI samples. The first runs of PEI showed very poor peak behaviour: multiple peaks were recorded at very low molecular weight, which was attributed to strong absorption on the column (data not shown). Over time and after switching between applications, the chromatograms for PEI improved such that useful results were obtained. This behaviour may be attributed to some deactivation of the active silanol sites on the columns over time, upon exposure to dibutylamine and other modifiers, for example. Table 2 displays the result of testing (average of five injections) obtained using “aged” columns. Although statistical t-tests show significant differences between SEC and UHPSEC data, the differences in absolute values are not very large. Considering that - according to our experience - even conventional SEC of PEI suffers from reproducibility issues, the obtained results are in good agreement.
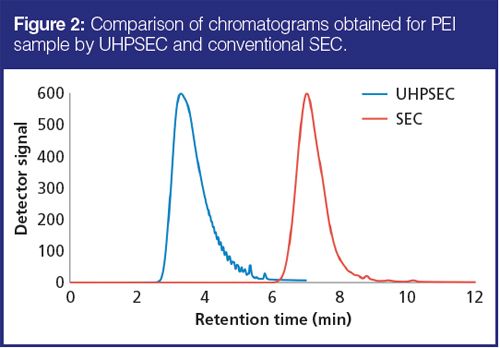
Chromatograms obtained with SEC and UHPSEC are shown in Figure 2. The peaks are depicted on the same time scale. Even though the UHPSEC run takes only 6 min (versus 12 min for SEC), small PEI oligomers are much better resolved at these conditions, demonstrating that higher separation efficiency is achieved simultaneously with a twofold decrease in analysis time.
Polyphenylene Ether (PPE):
PPE is known to be a difficult-to-analyze polymer because of the presence of pendant amine groups. It requires the addition of a modifier when running on standard styrene-divinylbenzene materials in conventional SEC. It was analyzed here using chloroform with 100 ppm of dibutyl amine (DBA) as a modifier to reduce interaction of the pendant amines with the column packing material. The recorded Mw data in Table 2 was approximately 10% lower with UHPSEC than that achieved with standard SEC. It is likely that the absorption effect is encountered and multiplied when using the silica type phase in UHPSEC. Although further stabilization was not investigated, it may be worthwhile to use alcohol or higher concentrations of DBA in the eluent. It is important to note that even though some adsorption effect has been observed, the repeatability of the separations was very good and the results are useful practically.
Polybutylene Terephthalate (PBT):
PBT is a polyester that is hard to dissolve in most chromatographic solvents; hexafluoroisopropanol (HFIP) needs to be added to the mobile phase and to the sample solvent. PBT is known to contain a number of different end-groups (8). Particularly acidic end-groups may adversely affect SEC separations and cause adsorption and ionic interactions (9). As a result of uncontrollable adsorption in UHPSEC under the same conditions as used in standard SEC (10% HFIP in chloroform), PBT was analyzed by UHPSEC using a mixture of 5% HFIP and 0.1% formic acid in chloroform, which was found to prevent column interaction to a certain extent. The addition of small amounts of alcohol (butanol) to the mobile phase and an increase in temperature was observed to further reduce adsorption effects. It is important to note that, in contrast to the other polymers, the mobile phase conditions used here are different for UHPSEC and SEC. The hydrodynamic volume of both PBT polymers and PS standards used for calibration may be different in these eluents, leading to differences in the molecular weight values. The results for this polymer show the most differences of the four types of polymers tested. However, because of the good repeatability of the measurements, the data was found to be suitable for particular applications (for example, for comparison of samples).
Repeatability:
The repeatability of consecutive UHPSEC measurements was tested by recording at least five injections of each polymer and evaluating the relative standard deviation (RSD) of the measured Mw and Mn values. In all cases the RSD value was below 1% and it was statistically equal to the RSD provided by the SEC instrument (according to the f-test). These results confirmed that even though the stationary phase for UHPSEC has different chemistry and may sometimes exhibit some secondary interactions with analytes, it supplies stable and reliable data. This is an important conclusion to draw, and proved that although measured molecular weights for SEC and UHPSEC are somewhat different in absolute values, UHPSEC is able to detect small differences between samples as accurately as the legacy SEC technique. This is an essential need for many practical applications.
Degradation of Polymers:
When large macromolecules are travelling through a packed column with a solvent flow, the polymer chains may undergo degradation by scission because of high shear and high elongational rates generated on the chromatographic columns (10). The smaller the stationary phase particles the larger the forces that are produced on the column, and the shorter the polymer chains for which the degradation starts to occur. Therefore, the application of small particles in UHPSEC will make this effect more severe. Obviously, the degradation of polymers during analysis is highly undesirable and it will lead to the polymer molecular weight being underestimated. Experimental work by Hofe
et al.
has shown that no degradation of PS with molecular weights up to 6 MDa occurred at UHPLC conditions (11). This effect was studied in detail for UHPSEC with sub-2-µm particles (12). It was found that at common UHPLC flow rates (linear velocities up to 7 mm/s) the degradation occurred only for large PS chains (above 3 MDa). This was only a slightly lower molecular weight for onset of the degradation compared to that reported for conventional SEC (10). Therefore, even though the UHPLC conditions increase stress on the macromolecules during separation, its adverse effect on the polymer chain is less significant than previously suggested. The onset of degradation in terms of molecular weight is highly dependent on the type of polymer and it can occur earlier for ridged polymer chains. Degradation can be suspected when the measured molecular weight is lower than expected, and when the molecular weight decreases while polydispersity increases with increasing flow rate. PC, PEI, PPE and PBT polymers (see molecular weight values in the previous section) were analyzed with UHPSEC at different flow rates up to 2 mL/min at pressures up to 600 bar; no evidence of degradation for these polymers was obtained.
Practical Benefits of UHPSEC
Separation Efficiency:
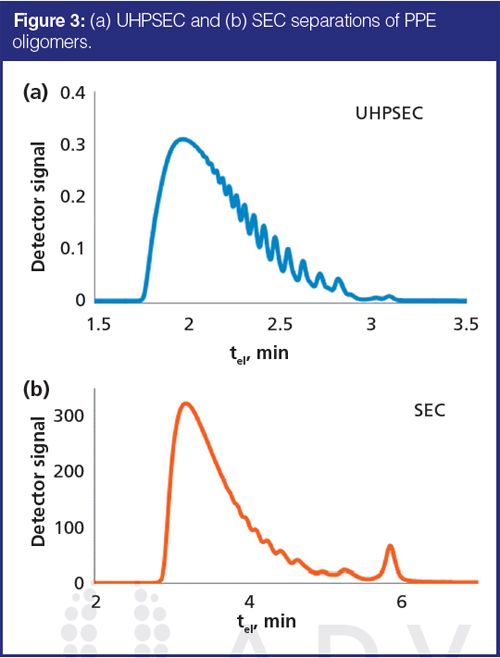
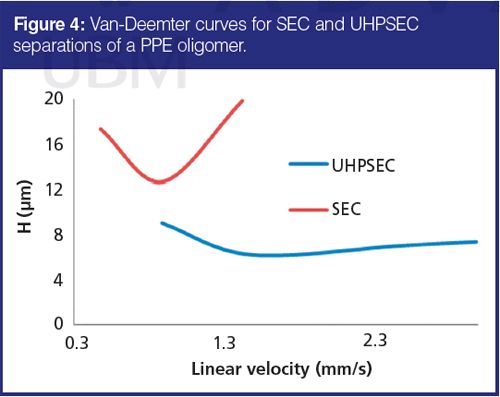
One of the main advantages of using columns packed with small particles at elevated pressures is decreased chromatographic band broadening, and therefore, higher separation efficiency. This can be clearly observed when analyzing oligomeric samples. In Figure 3 a comparison of SEC and UHPSEC separation of PPE oligomer is shown. Even though SEC separation was performed using a column specially designed for oligomer separations, it is obvious that the UHPSEC column provided higher resolution in the low-molecular-weight region. It should be noted that more efficient UHPSEC separations were obtained in half the time versus that of SEC. The van Deemter curve constructed for one of the separated oligomers (Figure 4) confirmed expected low plate height values and a more shallow slope for UHPSEC compared with SEC.
Analysis Speed:
According to theoretical studies, small particles bring the most advantages when used at high flow rates, and under these conditions they provide the best trade-off between analysis speed and efficiency compared to other available options (such as monoliths, core–shell particles, etc.) (13). Fast SEC separations are especially useful in quality control laboratories where numerous samples need to be analyzed quickly or for high-throughput screening of new products. Another important application of fast SEC is as the second dimension of comprehensive two-dimensional liquid chromatography (LC×LC) for polymer analysis. The main requirement for the second dimension separation is a very short analysis that can provide sufficient separation efficiency. The faster the second dimension run the better the first dimension separation can be maintained and the shorter the total separation time is. Fast SEC columns that are typically used in polymer LC×LC enable 2 to 3 min second dimension separations that result in a total LC×LC analysis time of several hours, rendering this technique impractical for routine applications. Speeding up SEC beyond 2 min enables completion of LC×LC in a reasonable time (for example, within 1 h) while providing high-quality analytical data (14). The applicability of UHPSEC technology was evaluated for very fast separations of engineering plastics. The combination of a factor of three reduction in column length along with a doubling of linear velocity resulted in a reduction of run time from 6 min for the normal UHPSEC to 1 min for fast UHPSEC. Under these conditions the columns were operated close to the maximum recommended pressures. High-speed UHPSEC results were compared with UHPSEC data obtained at normal flow rates and with data provided by conventional SEC for different polymers (Table 2). Fast UHPSEC data for PC were very close to that of conventional SEC and of “normal” UHPSEC, confirming that no significant interactions with stationary phase occurred for this polymer. For the other three polymers the data obtained at high flow rates with the shorter columns were somewhat different. This can be explained by different column history, which has resulted in different amount of free silanol groups, and by small changes in the secondary interactions as a result of different flow rates (as a result of a higher amount of heat generated on the column at higher flow rates). Overall the results were very stable and repeatable and the absolute differences in measured molecular weights were not dramatic. This confirms the potential of UHPSEC for ultra-fast polymer separations, especially for LC×LC applications where relative differences between samples are usually more important than the absolute molecular weights.
Solvent Exchange:
The use of a silica-organic hybrid stationary phase brings an important advantage for the UHPSEC technique. This is a ridged material that hardly swells or shrinks in organic solvents offering the option of easily and quickly exchanging mobile phases without damaging the column and without any adverse effects on chromatographic separations. It provides the option of using a single SEC system and a single set of columns for all types of polymers.
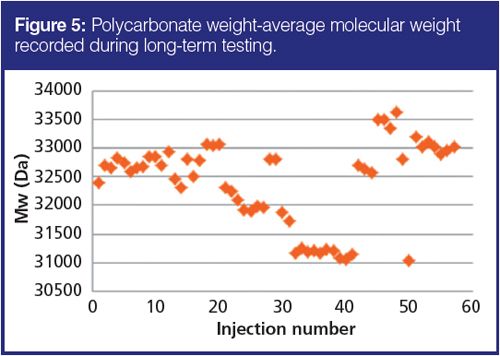
To evaluate the robustness and the repeatability of separations after changing solvents and switching between applications, PC samples were regularly injected and their measured molecular weights were compared. In between PC tests, various samples – including a range of PBTs, PEIs, and PPEs and corresponding solvent compositions – were run on the system. The instrument was operated under chloroform, dichloromethane, and HFIP with different organic modifiers at different temperatures (up to 50 °C). The same set of columns was used for all measurements. The results of PC molecular weight measurements over the period of three months are shown in Figure 5. The obtained weight average molecular weights were repeatable within 5% of RSD, demonstrating that multiple changes of solvents, temperatures, and sample types did not affect the polymer separations. The data is very promising for research and development work where a large variety of solvents and repeated switching between applications may be encountered.
Conclusions
It was demonstrated that columns packed with small (sub-3-µm) particles operated at elevated pressures could provide very efficient and very fast separations of polymers. As a result of the different stationary phase chemistry used for UHPSEC, the molecular weights obtained with this technique were somewhat different from those obtained on standard polystyrene divinylbenzene SEC columns, particularly for polymers containing polar (end-)groups. However, for most polymers tested in this study the absolute molecular weight differences were not very large and were comparable to the variations that could be obtained using different types of conventional SEC columns. Using mobile phase modifiers can further minimize the differences. It must be noted that for applications where consistency of data with the existing SEC method is important, the applicability of UHPSEC needs to be assessed on a case-by-case basis. These results suggest that the data is more comparable for polymers with relatively low polarity. Although high-resolution oligomer analysis is an important UHPSEC application, the main advantage of UHPSEC is likely to be in ultrahigh-speed polymer separations where no other currently available technique can offer a better solution. SEC separations that can be achieved within 1 min, alongside some reduction in the amount of organic solvent used for analysis, makes this technique very attractive for industrial applications where large number of samples need to be analyzed on a daily basis. In addition, a two- to threefold decrease in analysis time compared to a currently used fast SEC approach offers great improvements for two-dimensional separation of polymers. Fast UHPSEC applied in the second dimension will enable an even higher sampling rate in LC×LC and will allow elucidation of finer differences in polymer composition in a shorter timeframe. In terms of other parameters such as short-term and long-term precision and the onset of polymer degradation, UHPSEC was found to be comparable to conventional SEC. Even after multiple changes of mobile phases and switching between applications (typically avoided in conventional SEC), molecular weight results provided by UHPSEC were very stable. Using trimethyl silane-bonded silica-organic hybrid material as a stationary phase for UHPSEC offers sufficient mechanical stability even at elevated pressures while reducing secondary interaction effects on the column, thereby making fast and efficient SEC separations possible. Although such UHPSEC columns are currently available from a single manufacturer, many results described in this study are applicable for adsorption LC of polymers for which different UHPLC stationary phases could be used. Moreover, SEC can also be achieved on columns that are originally designed for interactive LC, when sufficiently strong mobile phase is applied to elute the polymer sample (2). Obviously, in this case, the separation mechanism is more difficult to control and the molecular weight range will be limited as a result of a lack of available pore sizes.
Acknowledgements
The authors would like to acknowledge Noor Abdulhussain who explored potential of UHPSEC for separations of engineering plastics during her internship at SABIC. The authors express special gratitude to Waters Corporation and in particularly to Damian Morrison, Ed Bouvier, Tanya Tollifson, and Michael O’Leary for providing an opportunity to evaluate a UHPSEC instrument.
References
1. J.J. van Deemter, E.J. Zuiderweg, and A. Klinkenberg,
Chem. Eng. Sci.
5
, 271–289 (1956). 2. E. Uliyanchenko, P.J. Schoenmakers, and Sj. van der Wal,
J. Chromatogr. A.
1218
(11), 1509–1518 (2011). 3. M. Janco, J.N. Alexander, E.S.P. Bouvier, and D. Morrison,
J. Sep. Sci.
36
, 2718–2727 (2013). 4. H.G. Barth and G.D. Saunders,
LCGC LC Column Techn. Suppl.
s24
(4), 38–43 (2006). 5. D. Held, G. Reinhold, and P. Kilz,
The Column
6(6), 10–14 (2010). 6. J.T. Bendler,
Handbook of polycarbonate science and technology
(CRC Press, 1999). 7. S. Carroccio, C. Puglisi, and G. Montaudo,
Polym. Degr. Stab.
80
, 459–476 (2003). 8. X. Lou, J.L.J. van Dongen, H.M. Janssen, and R.F.M. Lange,
J. Chromatogr. A.
976
, 145–154 (2002). 9. B. Trathnigg,
Prog. Polym. Sci.
20
, 615–650 (1995). 10. H.G. Barth and F.J. Carlin,
J. Liq. Chromatogr.
7
(9), 1717–1738 (1984). 11. T. Hofe, G. Reinhold, and J. McConville,
Chrom. Today
4
(4), 18–25 (2011). 12. E. Uliyanchenko, Sj. van der Wal, and P.J. Schoenmakers,
J. Chromatogr. A
1218
(39), 6930–6942 (2011). 13. D. Guillarme, J. Ruta, S. Rudaz, and J.L. Veuthey,
Anal. Bioanal. Chem.
397
(3), 1069–1082 (2010). 14. E. Uliyanchenko, P.J. Cools, Sj. van der Wal, and P.J. Schoenmakers,
Anal. Chem.
84
(18), 7802–7809 (2012).
Elena Uliyanchenko
obtained her MSc degree in chemical engineering from Volgograd State Technical University, Russia, and her PhD degree in analytical chemistry from the University of Amsterdam, The Netherlands. Her PhD work focused on the application of ultrahigh-pressure liquid chromatography and two-dimensional liquid chromatography to the analysis of polymers. She is currently a Senior Scientist at SABIC, The Netherlands, where she leads a chromatography team and works on applying state-of-the-art chromatographic and mass-spectrometry techniques to the analysis of engineering thermoplastics.
Christian Wold
is a Chief Scientist at SABIC, The Netherlands. He gained his PhD in applied analytical sciences from the University of Newcastle, Australia. He has 14 years of experience working on the instrumental analysis of polymers using a variety of techniques and with a focus on chromatography.


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











