Surfactant-Mediated Extractions, Part 1: Cloud-Point Extraction
LCGC Europe
Cloud-point extraction (CPE) manipulates temperature and surfactant concentration to move aqueous solutes into a micelle phase for separation. Although CPE has been around for some time, it is still considered an emerging technique. Much of the development, and most applications, of CPE have dealt with extraction and preconcentration of inorganic solutes. More recently, attention has turned to the use of CPE in the isolation of organic solutes. This month, we review how CPE works and focus on applications for extracting organics.
Douglas E. Raynie, Sample Preparation Perspectives Editor
Cloud-point extraction (CPE) manipulates temperature and surfactant concentration to move aqueous solutes into a micelle phase for separation. Although CPE has been around for some time, it is still considered an emerging technique. Much of the development, and most applications, of CPE have dealt with extraction and preconcentration of inorganic solutes. More recently, attention has turned to the use of CPE in the isolation of organic solutes. This month, we review how CPE works and focus on applications for extracting organics.
With the rapid development of sample preparation technologies since the mid-1970s, one sometimes overlooked technique that is getting renewed interest for chromatographic sample preparation is cloud-point extraction (CPE). CPE is the most widely used in a family of surfactant-based methods and was introduced in 1976 by Mitura, Ishii, and Watanabe (1). Initially, applications in the extraction of metal ions and, later, hydrophilic proteins seemed to predominate. But in the past several years, attention has shifted to small molecule organics, warranting a look at CPE as a promising, environmentally benign means for chromatographic sample preparation.
What Are Micelles?
Surfactant molecules generally possess a hydrophilic head group attached to a hydrophobic tail. At water–air or water–organic interfaces (or with other polar solvents besides water), these molecules align so that the hydrophilic portion is directed towards the aqueous component of the mixture. As concentration increases, the dispersed hydrophobic chains self-assemble into colloidal-sized clusters (2). When a concentration known as the critical micelle concentration (CMC) is reached, these colloidal aggregates are in dynamic equilibrium with surfactant monomers in the bulk aqueous solution and are called micelles. In these micellar colloids, water is the continuous phase and the aggregated surfactant molecules are the dispersed phase. Micelles are typically approximately spherical, but may also have other shapes depending on the nature of the surfactant and the solution properties. Reverse micelles, in which the hydrophobic chains are on the outside, are found with nonpolar solvents.
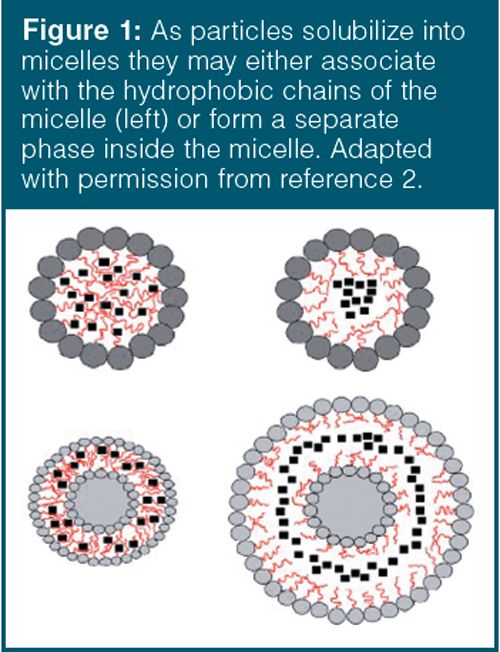
The degree of aggregation, or micelle size, is dependent on the nature of the surfactant (including the identity, or structure, of the hydrophilic and hydrophobic groups), solutes (especially electrolytes), nature of the solvent, temperature, and pH. Micelles will only form above the critical micelle temperature. Micelles in water-based solutions may solubilize hydrophobic compounds or those materials with limited water solubility to a much greater extent than in pure aqueous solutions. The hydrophobic solutes partition into the inner core of the micelle, as depicted in Figure 1. Similarly, reverse micelles dissolve hydrophilic substances into the micelle from a nonpolar organic solvent. Polar compounds may migrate to the centre of the micelle, associate with the surfactant’s polar head groups of the surfactant, or associate with the micelle hydrophobic chains. These compounds are solubilized through the polar region by electrostatic, π-ion, or hydrogen-bonding interactions.
For the “cloud-point system” in CPE, the phase transfer of nonionic surfactants from a clear homogenous solution to the cloudy colloidal system, and then a biphasic system upon increasing the temperature above the ‘‘cloud-point temperature’’ (CPT) of the surfactant is used (3). The term cloud point refers to the light scattering created by the formation of a colloidal system, the Tyndall effect.
Mechanism of Extraction
CPE is performed by adding a surfactant solution to the sample at levels exceeding the CMC, allowing the formation of micelles. As the analytes dissolve and partition into the micelles, two immiscible isotropic phases form. The first of these phases is the surfactant-rich coacervative phase, which contains the extracted analyte. The bulk aqueous phase is in equilibrium with the coacervative phase. It is important to note that in CPE, only nonionic or zwitterionic surfactants are used in forming the colloidal micelles and temperature (the CPT) is used to induce the phase separation (2).
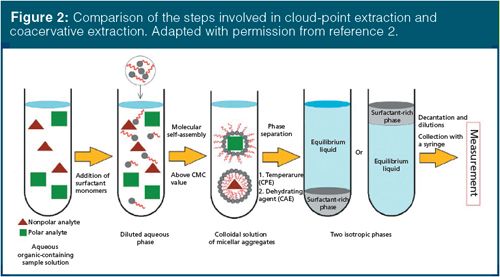
CPE is not to be confused with coacervative extraction (CAE), even though these terms are sometimes used interchangeably in the literature. The physical and chemical processes in CPE and CAE are similar, as diagrammed in Figure 2. In CPE, temperatures above the CPT are used to induce the phase separation, while in CAE, salts, organic solvents, pH, or other factors are used. Because of this difference in the conditions regarding how the phase separation occurs, CAE may use anionic or cationic surfactants. With the resulting charged micelles, temperature alone cannot overcome the electrostatic repulsions that prevent phase separation, and decreased temperature (rather than above the CPT) is used. CAE and other surfactant-based extractions, like aqueous surfactant two-phase (ASTP) extraction, which uses mixtures of ionic surfactants, will be the subject of a future “Sample Preparation Perspectives” column.
CPE involves three fundamental steps:
- analytes present in the original matrix associate within the micellar aggregates;
- increasing the temperature to above the CPT, which causes separation of the coacervative phase from the bulk aqueous phase; and
- phase separation, often by centrifugation and decanting.
The nonionic surfactants used in CPE are often polyoxyethylene-based, and commonly used surfactants include:
- Triton X-100 (CPT = 66 °C)
- Triton X-114 (CPT = 25 °C)
- Brij 30 (CPT = 2 °C)
- Brij 35 (CPT > 100 °C)
- Brij 56 (CPT = 69 °C)
- Brij 97 (CPT = 72 °C)
- Tween 80 (a sorbitol-based nonionic surfactant) (CPT = 65 °C).
CPT depends on the surfactant used and covers a broad temperature range, as shown above. Nonionic surfactants generally have a lower CPT than zwitterionic surfactants. The CPT may also vary with surfactant concentration and dissolved solutes, especially salts. The coacervative phase is usually cooled after the centrifugation step before decanting to collect the extract. Solute partitioning into the micelles from the bulk aqueous phase is primarily driven by hydrophobic interactions (van der Waals forces), with dipole–dipole and hydrogen bonding interactions playing separate roles and having secondary effects (3). Similar effects are observed in
liquid–liquid extraction into nonpolar solvents. Extraction kinetics and achievement of the equilibrium of partitioning is rapid, as fast as 2 min (4). To accomplish such rapid extractions, with near 100% extraction efficiency, temperatures 15–20 °C greater than the CPT are used (5–7).
Advantages and Limitations of CPE
CPE involves just a few manual steps and uses standard glassware and equipment found in most laboratories - namely pipettes, flasks, heating plates, and a centrifuge. Hence, specialized instrumentation or extraction supplies are not needed. In most cases, cleanup of the extracted sample before chromatographic determination is not necessary. The surfactants are fairly inexpensive and have low flammability. As mentioned, quantitative yields are obtained in short times and several samples may be processed at once.
At the same time, there are some limiting factors to consider. The surfactants used may cause analytical interferences and issues with detection limits, especially if the analytes cannot be effectively isolated from the surfactants, depending on the analysis. Extraction efficiencies decrease with increasing solute polarity and with highly volatile or thermally unstable compounds.
Applications
Although the initial applications of CPE were directed towards metal ions and other hydrophilic compounds, recent attention has turned to small organic molecules, especially. The extraction of priority pollutants from water, solid, and biological samples by CPE has been established (4). These include phthalates, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, polychlorinated dibenzodioxins and dibenzofurans, chlorophenols, and organochlorine pesticides.
The CPE of triazine herbicides in milk was reported by Liu and colleagues (8) as an example of food analysis. Atrazine, cyanazine, simazine, and simetryn were isolated from 10 mL of milk mixed with Triton X-100, glacial acetic acid, and sodium sulphate then diluted with water. Following centrifugation at 5 °C, the supernatant was filtered and the acidity was adjusted to pH 5. Following a 30-min incubation at 60 °C to cause phase separation, methanol was added to the coacervative phase, which was then filtered and characterized by high performance liquid chromatography with ultraviolet absorbance detection (HPLC–UV). Detection limits around 5–10 mg/L with recoveries of 70.5–96.9% and a linear range of 50–2000 mg/L were reported.
Other interesting applications are in drug-related studies. Benzodiazepines were extracted from medicinals with Triton X-114 at pH 6 at 40 °C and 30 min (9,10). Lorazepam was recovered at 52.7% and alprazolam at 67.0% with HPLC using diode-array detection (DAD). The preconcentration of β-lactam antibiotics with CPE was accomplished (11). Even more interesting are the myriad investigations into the determination of the active compounds in Chinese medicinal herbs (12–15).
Conclusion
Several review articles, including those that provided the basis for this column, establish the wide utility of CPE to the analytical extraction of small organic molecules (2–4,16–18). As greener, faster, and more efficient extraction methods are being investigated for the analytical laboratory, the use of CPE warrants a look.
References
- J. Mitira, H. Ishii, and H. Watanabe, Bunseki Kagaku25, 808–809 (1976).
- A. Melnyk, J. Namiesnik, and L. Wolska, TrACTrends Anal. Chem.71, 282–292 (2015).
- A.S. Yazdi, TrAc Trends Anal. Chem. 30, 918–929 (2011).
- S. Xie, M.C. Paau, C.F. Li, D. Xiao, and M.M.F. Choi, J. Chromatogr. A1217, 2306–2317 (2010).
- A. Santalad, S. Srijaranai, R. Burakham, J.D. Glennon, and R.L. Deming, Anal.Bioanal. Chem.394, 1307–1317 (2009).
- F. Merino, S. Rubio, and D. Pérez-Bendito, J. Chromatogr. A962, 1–8 (2002).
- X. Liu, X.-H. Chen, Y.-Y. Zhang, W.-T. Liu, and K.-S. Bi, J. Chromatogr. B856, 273–277 (2007).
- T. Liu, P. Cao, J. Geng, J. Li, M. Wang, M. Wang, X. Li, and D. Yin, Food Chem.142, 358–364 (2014).
- K. Madej, K. Persona, and M. Nizio, Acta Chromatogr.25, 97–110 (2013).
- K. Persona, K. Madej, P. Knihnicki, and W. Piekoszewski, J. Pharm. Biomed. Anal.113, 239–264 (2015).
- C. Kukusamude, A. Santalad, S. Boonchiangma, R. Burakham, S. Srijaranai, and O. Chailapkul, Talanta81, 486–492 (2010).
- J. Zhou, X.L. Sun, and S.W. Wang, J. Chromatogr. A1200, 93–99 (2008).
- C. Sun and H. Liu, Anal. Chim. Acta612, 160–164 (2008)
- X.J. Wang, H.T. Lv, H. Sun, W.J. Sun, L. Liu, P. Wang, and H.X. Cao, Arzneim.-Forsch.58, 336–341 (2008).
- K. Kiathevest, M. Goto, M. Sasaki, P. Pavasant, and A. Shotipruk, Sep. Purif. Technol.66, 111–117 (2009).
- M. Moradi and Y. Yamini, J. Sep. Sci.35, 2319–2340 (2012).
- Z.S. Ferrera, C.P. Sanz, C.M. Santana, and J.J. Santana Rodriguez, TrAC Trends Anal. Chem.23, 469–479 (2004).
- R. Carabias-Martinez, E. Rodriguez-Gonzalo, B. Moreno-Cordero, J.L. Perez-Pavon, C. Garcia-Pinto, and E. Fernandez Laespada, J. Chromatogr. A902, 251–265 (2000).
“Sample Prep Perspectives” editor Douglas E. Raynie is an Associate Research Professor at South Dakota State University, South Dakota, USA. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee. Direct correspondence about this column should be addressed to “Sample Prep Perspectives”, LCGC Europe, Hinderton Point, Lloyd Drive, Ellesmere Port, CH65 9HQ, UK, or email the editor-in-chief, Alasdair Matheson, at amatheson@advanstar.com

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.












