UHPLC, Part 1: Perspectives and Instrumental Features
LCGC Europe
This instalment highlights historical perspectives on the development of ultrahigh-pressure liquid chromatography (UHPLC) into a modern high performance liquid chromatography (HPLC) platform and describes the important instrumental features common to most commercial equipment.
Michael W. Dong, Perspectives in Modern HPLC Editor
This instalment highlights historical perspectives on the development of ultrahigh-pressure liquid chromatography (UHPLC) into a modern high performance liquid chromatography (HPLC) platform and describes the important instrumental features common to most commercial equipment.
This instalment of “Perspectives in Modern HPLC” is the first entry of a three-part series on ultrahigh-pressure liquid chromatography (UHPLC), the most important recent development of modern high performance liquid chromatography (HPLC). This instalment focuses on perspectives and instrument features of UHPLC systems on the market. The second instalment will describe the benefits of UHPLC in highâspeed and high-resolution analysis, and the third will address its potential issues and operating nuances as well as how to get started in UHPLC.
Historical Perspectives and Some Personal Recollections
Modern HPLC has a history of about 50 years (1,2). For the first four decades, the pressure limit of most commercial HPLC systems was standardized at 6000 psi or 400 bar. There was no magic formula or regulations regarding this pressure limit, except that it was a good match with the columns packed with 10- or 5-µm particles during the time (3). The performance for routine analysis was capped at a level of ~20,000 plates or peak capacities of ~200. The HPLC ultraviolet–visible (UV–vis) detectors typically had flow cells with a volume of ~10 µL, which worked well for analyses using 4.6-mm i.d. columns (2). There were research efforts with systems built for higher pressures, notably those performed by Bidlingmeyer and colleagues in the 1960s using system pressures up to 50,000 psi (4). However, there was little need for such higher-pressure systems at that time.
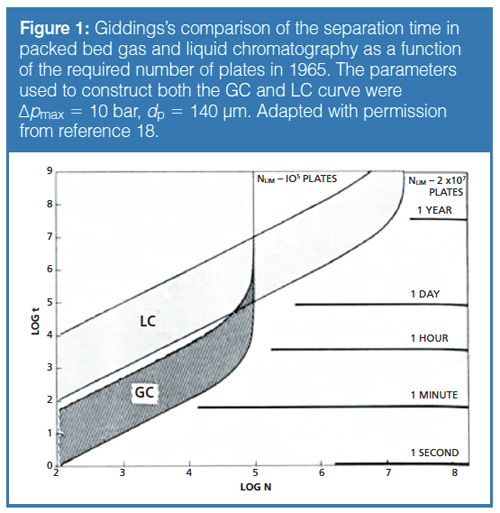
In the early 1980s with the debut of 3-µm silica particles, it was possible to perform high-speed LC with short LC columns in combination with lowâdispersion systems (total 4σ instrumental bandwidth of ~6 µL with 1.4-µL UV flow cells) as demonstrated by DiCesare and colleagues (5–7). An example of a fast LC gradient analysis of a sample of human serum in 90 s published in 1984 is shown in Figure 1.
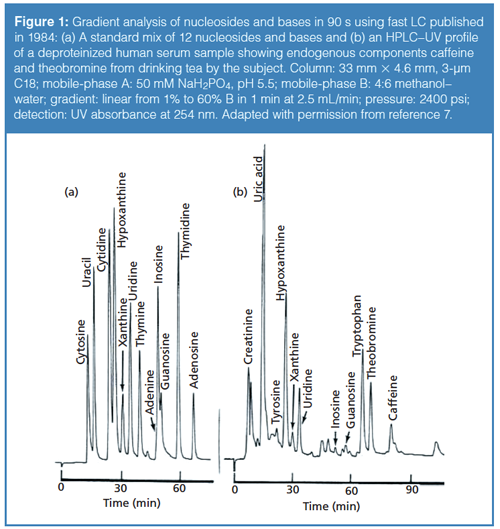
When sub-3-µm and sub-2-µm silica particles became available in the 1990s, there were greater incentives for designing higher-pressure systems as had been initially postulated by Martin and Synge in 1941 (8). They stated that “the smallest HETP [height equivalent to the theoretical plate] should be obtained by using very small particles and a high pressure difference across the length of the column” (8). In 1997, Professor James Jorgenson at the University of North Carolina at Chapel Hill published the pivotal proof-of-concept research (see Figure 2) (9,10), which led to the development of commercial UHPLC systems a few years later.
I heard of Jorgenson when he developed the first capillary electrophoresis instrumentation as a graduate student under Milos Novotny at the University of Indiana. Novotny was a consultant for the instrument company I worked for at that time and he proudly showed an electropherogram from his graduate student (Jorgenson) at a company consulting meeting in 1981. Although the performance of UHPLC demonstrated in 1997 was truly impressive (9), its impacts could only be realized with the availability of commercialized UHPLC systems and columns. This introduction came at Pittcon 2004 in Chicago, Illinois, USA, at which Waters Corporation announced the Acquity UPLC system with an upper pressure limit of 15,000 psi or 1000 bar (11,12). In the same year, Jasco announced a 15,000-psi UHPLC system (X-LC).
In the early years (2004–2007), UHPLC was embraced by research scientists for high-throughput screening, though there were questions on its application in regulatory testing (13). I recall attending an unusual webinar given by a pharmaceutical scientist who discussed whether this latest technology was a “tool box” or a “toy box”. Others argued that expensive equipment was not needed because one could use superficially porous particle (SPP) columns or highâtemperature LC (14). In May 2010, I was invited to give an UHPLC presentation at a dinner meeting in California, which later turned out to be a debate on UHPLC versus highâtemperature LC (15). These arguments against UHPLC were not valid, since both LC with SPP columns and high-temperature LC can be performed on UHPLC systems with superior results, because they are options rather than alternatives (14). By that time, Agilent (model 1290), Thermo Fisher Scientific (Acela and Dionex Ultimate), Shimadzu (Nexera), and PerkinElmer (Flexar) had introduced their own versions of UHPLC products, and the debate was essentially over with fewer skeptics voicing objections or contrarian theories. As improved second-generation UHPLC systems have debuted, UHPLC has gained acceptance in both research and industry as a modern HPLC platform capable of higher speed and resolution for applications in all market sectors (12,16–22).
As a chromatography specialist, it was an exciting time for me to witness the birth and eventual universal adoption of UHPLC. I often wondered why it took five decades to see this very moderate three- to fivefold increase of HPLC performance. Was it the conservatism of regulatory testing or possibly some other reason, such as the slow instrumental development cycles? In contrast, DNA sequencing technologies have increased their efficiencies about 100,000-fold in just two decades.
Instrumental Features of UHPLC Systems
Modern UHPLC systems have three common instrumental features. Besides having higher pressure limits than conventional HPLC systems, they also have lower system dispersion and smaller dwell volumes. Readers are also referred to a recent review article in Analytical Chemistry for additional details of UHPLC system design (23).
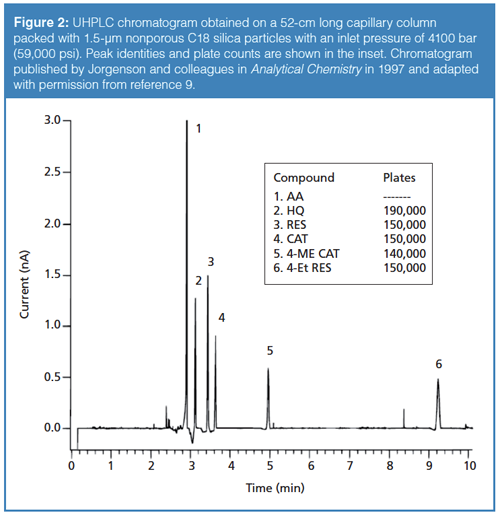
System Pressure: Figure 3 illustrates the benefits of using columns packed with small particle sizes (dp), which then requires higher system pressures. In the four comparative chromatograms operated under identical mobile-phase conditions and using columns of identical dimensions (50 mm i.d.) but packed with decreasing dp of 10, 5, 3.5, and 1.7 µm, substantial increases in resolution and sensitivity (peak heights) are realized. The theoretical plate number (N) increase from 2500 to 14,700 (N = L/2dp) (2). However, with a sixfold decrease in dp (from 10 to 1.7 µm), the operating pressure (ΔP) is increased 62 or 36 times since ΔP is inversely proportional to dp2 (2). The optimum flow velocity is also inversely proportional to dp, so ΔP is actually inversely proportional to dp3 at optimum flow rate.
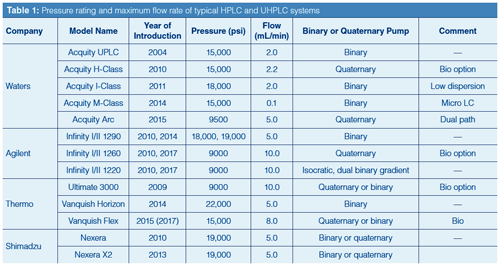
Systems that offer very high system pressure capability are advantageous to allow the use of columns packed with very small dp to generate ultrafast separations, however, there is a clear practical limit imposed by engineering or manufacturing capability, long term system reliability, and equipment cost. Table 1 shows the current offerings of HPLC and UHPLC systems from major manufacturers with their year of introduction, upper pressure limits (in psi), the availability of binary or quaternary pump, and the maximum flow rates. Note that most UHPLC systems have maximum pressure limits ranging from 15,000 to 22,000 psi, with several lower-cost intermediate-pressure or dual-path systems rated at
9000–10,000 psi.
Although UHPLC systems have been defined as a system with a pressure rating >15,000 psi, there is no absolute consensus as to what the pressure limit should be. Jorgenson expressed his opinion many times at national meetings that the term UHPLC should be reserved for instruments operating above 25,000 psi. If adopted, his definition would recategorize all current systems-downgrading them to a less attractive category such as very-high-pressure liquid chromatography or VHPLC. Clearly, no one has volunteered for such a downgrade.
Note that although designing a UHPLC pump that can run reliably and precisely at >15,000 psi was the initial requirement, having an autosampler that can withstand such pressures with the ability to inject very small volumes precisely poses additional challenges.
Maximum Flow Rate: As shown in Table 1, the maximum flow rates of most UHPLC systems are also lower than those of conventional HPLC systems (typically at 10 mL/min). For some obscure reason, conventional HPLC systems were designed for both analytical and semipreparative use, even though no analyst in his or her right mind would ever use an analytical system for preparative use because of the high risk of system contamination. In reality, a maximum flow rate of 2 mL/min is typically adequate, except for system priming when higher flow rates are advantageous.
Column Use: The first columns designed specifically for use with the Waters Acquity UPLC system were Acquity 1.7-µm C18 and C8 columns packed in 2.1-mm and 1.0-mm i.d. formats. The marketing slogan of “Acquity UPLC for Acquity columns”, or vice versa, was quite attractive in 2004 when there was little market competition. However, most laboratory scientists would like to use their expensive UHPLC systems with all kinds of columns including those for existing HPLC methods using 4.6âmm i.d. columns packed with largerâdiameter particles. Backward method compatibility has become an important marketing benefit for those UHPLC systems that are more amenable to running conventional HPLC methods-for example, those specifying longer columns, higher flow rates, and larger injection volumes (14).
System Dispersion: All modern UHPLC systems are designed to be low-dispersion systems, reducing the extracolumn bandbroadening of peaks when using narrower columns (for example, 2.1 mm i.d.). System dispersion is related to the fluidic volumes of the system experienced by analyte molecules, from the injector and connection tubing to the detector flow cell. The void volume of the column and the tubing volumes before the injector or after the detector flow cell are not part of the extracolumn volumes responsible for system dispersion (2).
How to Measure System Dispersion:
One way to measure system dispersion in a HPLC–UV system is by replacing the column with a zero-dead-volume union, and making a small volume injection of a chromophoric analyte (for example, caffeine at 0.1 mg/mL) at a low flow rate (such as 0.4 mL/min) as shown in Figure 4. When a small plug of sample (for example, 1 µL) is injected under these conditions, instead of emerging as a 1-µL-broad rectangular band in the UV detector, the sample band immediately broadens upon injection because of Poiseuille flow, a parabolic flow profile resulting from friction along the tubing wall in the internal channels of the injector, connecting tubing, and UV detector flow cell. Thus, a peak like the one shown in Figure 4 is generated, which is known as an instrument bandwidth (IBW) peak. A 4σ IBW peak width can be estimated by drawing two tangent lines from the widthâat-half-height inflection points, similar to the procedure for calculating United States Pharmacopeia (USP) plate counts from the 4σ base width.
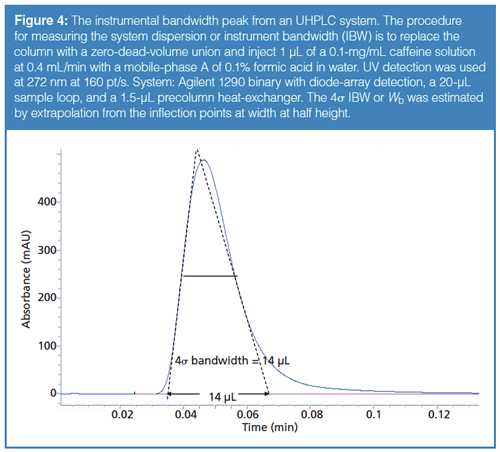
Table 2 lists system dispersion or IBW (4σ) of several common UHPLC systems measured using the procedure described above. Note that conventional HPLC systems often have 4σ IBW in the 30–60 µL range, while modern UHPLC systems have IBW in the 10–20 µL range. Very-low-dispersion systems (where IBW < 10 µL, such as with the Waters Acquity I-Class system) are needed to obtain close-to-theoretic column efficiencies for narrow and short columns that generate very small peak volumes, particularly for early-eluted peaks at low k under isocratic conditions (2,23).
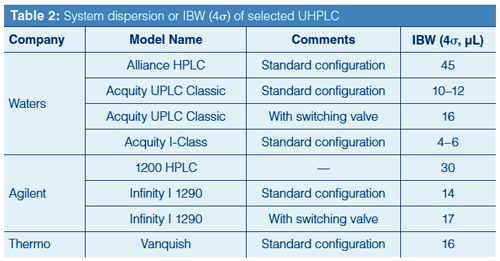
Some Fine Points on the Effect of System Dispersion: UHPLC systems with very low system dispersion are required for maintaining the highest column efficiencies under isocratic conditions (and for micro LC using columns with internal diameters less than 1.0 mm). This is typically achieved using a small injector sample loop, connection tubing with a very small internal diameter (for example, 0.003 in.), and a veryâsmallâvolume UV flow cell. Smaller HPLC–UV flow cells designed in the earlier years with shorter pathlengths (3–6 mm) were less desirable for sensitivity reasons. This problem was solved in UHPLC by using UV flow cells with total internal reflection allowing very small UV flow cells (for example, 0.5–1 µL with a 10-mm pathlength) to be built with little apparent sensitivity loss. Narrow-internal-diameter connection tubing (such as <0.003 in.) causes high back pressure (for example, 3000 psi at 1 mL/min without any column), and smaller sample loops (such as 10–20 µL) can be problematic for many conventional HPLC methods requiring large-volume sample injections (>20 µL).
Another nuance to remember is that one rarely requires very high resolution under isocratic conditions because most stability-indicating methods for potency and impurities determination are gradient methods where any system dispersion in front of the column is inconsequential (14). In reality, only postcolumn system dispersion (that is, the UV flowcell) is truly critical.
System Dwell Volume: The third important element of modern UHPLC systems is a low system dwell volume, which minimizes gradient delay times (2). Dwell volume is the fluidic volume in the HPLC system from the point of mixing two mobile-phase solvents to the head of the column. Dwell volumes are much lower in high-pressure mixing binary pumps (0.1–0.4 mL) where the mixing point is external to the pumps. Dwell volumes are substantially higher in low-pressure mixing pumps (0.5–1 mL for most quaternary pumps) since the mixing point is immediately after the proportioning valve and the internal volumes in the pump (pressure transducer, filter, and pulse-dampener) add substantial wash-out volumes. The system dwell volume can be measured by the procedure shown in the caption of Figure 5 using a mobile-phase B with a chromophoric additive (such as acetone) and a UV detector to track the gradient profile (2).
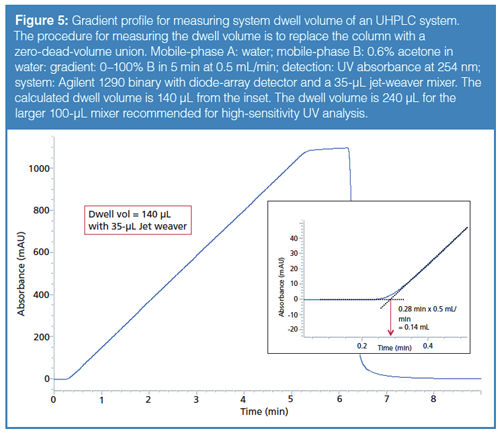
A system with low dwell volume is important for ultrafast gradient separations (high-throughput screening, in-process testing) and for lower flow rate applications (LC–mass spectrometry [MS] and micro LC). Note that a dwell volume of 1 mL will translate to a gradient delay time of 5 min at a flow rate of 0.2 mL/min. The dwell volumes of many UHPLC systems were measured and published by Fekete and colleagues (24) in 2014.
For high-pressure mixing binary pumps, external mixers are typically required to ensure adequate blending of the solvent and very stable baselines with UV detection (13). The optimal external mixer volume is dependent on the mixer type and pump design (for example, piston configuration [“dualâpistons” in parallel or in series], piston size, and stroke volume) (23). The selection of an appropriate optional mixer will be discussed in a later instalment.
Other Instrumental Features
There are secondary requirements for high-speed analysis in addition to system dispersion and dwell volume reductions, notably faster detector response and high data acquisition rates (for both UV and MS), and shorter autosampler cycle times (23). Also, many UHPLC systems are available in a biocompatible format (for example, built with titanium rather than stainless steel in the fluidic flow path) for better resistance to the high-salt mobile phases often used in protein separations.
Conclusion
This instalment is the first of three overviews on perspectives, benefits, and potential issues of UHPLC. This instalment highlights the historical perspectives on the development of UHPLC into a modern HPLC platform and describes the important instrumental features common to all commercial UHPLC systems.
Acknowledgements
The authors would like to thank Naijun Wu of Celgene, Ron Majors of LCGC, Rowan Moore and Chris Pohl of Thermo Fisher Scientific, Tom Walter of Waters, Tom Waeghe of MAC-MOD Analytical, Joe DiCesare and Wilhad Reuter of PerkinElmer, and Davy Guillarme of the University of Geneva for their technical and editorial inputs.
References
- C. H. Arnaud, Chem. & Eng. News94(24), 29–35 (2016).
- M.W. Dong, Modern HPLC for Practicing Scientists (Wiley, Hoboken, New Jersey, USA, 2006), chapters 2–3, 7.
- R.E. Majors, LCGC North Am.33(11), 818–840 (2015).
- B.A. Bidlingmeyer and L.B. Rogers, Anal. Chem.43, 1882–1883 (1971).
- J.L. DiCesare, M.W. Dong, and J.G. Atwood, J. Chromatogr.217, 369–386 (1981).
- J.L. DiCesare, M.W. Dong, and L.S. Ettre, Introduction to High-Speed Liquid Chromatography (Perkin-Elmer, Norwalk, Connecticut, USA, 1981).
- M.W. Dong and J.R. Gant, LCGC2, 294–302 (1984).
- A.J.P. Martin and R.L.M. Synge, Biochem. J. 35, 1358–1368 (1941).
- J.E. MacNair, K.C. Lewis, and J.W. Jorgenson, Anal. Chem.69, 983–989 (1997).
- J.E. MacNair, D. Patel, and J.W. Jorgenson, Anal. Chem.71, 700–708 (1999).
- U.D. Neue, M. Kele, B. Bunner, A. Kromidas, T. Dourdeville, J.R. Mazzeo, E.S. Grumbach, S. Serpa, T.E. Wheat, P. Hong, and M. Gilar, in Advances in Chromatogr. (CRC Press, Boca Raton, Florida, USA, 2009), pp. 99–143.
- “UHPLC: Where We Are Ten Years After its Commercial Introduction,” in Trends in Anal. Chem. D. Guillarme and M.W. Dong, Eds., 63, 1–188 (2014).
- M.W. Dong, LCGC North Am.25(2), 89–98 (2007).
- M.W. Dong, LCGC Europe26(11), 637–645 (2013).
- CASSS Bay Area Discussion Group Meeting May 2010.
- D. Guillarme and M.W. Dong, Am. Pharm. Rev.16(36), 38–43 (2013).
- M.W. Dong, LCGC North Am.34(4), 262–273 (2016).
- M.W. Dong, LCGC North Am.33(4), 254–261 (2015).
- M.W. Dong, LCGC Europe27(4), 213–220 (2014).
- M.W. Dong, LCGC Europe26(4), 216–221 (2013).
- D. Guillarme and J.-L. Veuthey, UHPLC in Life Sciences (Royal Society of Chemistry, Cambridge UK, 2012).
- Q.A. Xu, UHPLC and Its Applications (John Wiley & Sons, Hoboken, New Jersey, USA, 2013).
- J. De Vos, K. Broeckhoven, and E. Eeltin, Anal. Chem. 88, 262–278 (2016).
- S. Fekete, I. Kohler, S. Rudaz, and D. Guillarme, J. Pharm. Biomed. Anal.87, 105–119 (2014).
Michael W. Dong is a principal in MWD Consulting, focusing on training and consulting services in HPLC and UHPLC, pharmaceutical analysis, and drug quality. He was formerly a Senior Scientist at Genentech, a Research Fellow at Purdue Pharma, and a Senior Staff Scientist at Applied Biosystems/Perkin-Elmer. He holds a PhD in analytical chemistry from the City University of New York, and a certificate in biotechnology from U.C. Santa Cruz. He has more than 100 publications including a best-selling book on chromatography. He is an editorial advisory board member of LCGC North America. Direct correspondence about this column via e-mail to LCGCedit@ubm.com

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.