Evaluation of Retention and Selectivity Using Biphenyl Stationary Phases
LCGC Europe
In reversed-phase liquid chromatography (LC), C18 alkyl-based stationary phases have been the favourite of method developers. Phenyl stationary phases are an alternative that are thought to benefit from additional π-π mechanisms. Recently, there has been a growing interest in the use of phases based on the biphenyl moiety. This instalment of “Column Watch” looks at the retention mechanisms of biphenyl phases and contrasts them with those of more-common alkyl phases.
Daniel Shollenberger1, Hugh Cramer2, and David S. Bell3,1Analytical Research and Services Group, Arkema, King of Prussia, Pennsylvania, USA, 2MilliporeSigma, Bellefonte, Pennsylvania, USA, 3Column Watch Editor
In reversed-phase liquid chromatography (LC), C18 alkyl-based stationary phases have been the favourite of method developers. Phenyl stationary phases are an alternative that are thought to benefit from additional π-π mechanisms. Recently, there has been a growing interest in the use of phases based on the biphenyl moiety. This instalment of “Column Watch” looks at the retention mechanisms of biphenyl phases and contrasts them with those of more-common alkyl phases.
Although C18 alkyl-based phases remain the standard for reversedâphase liquid chromatography (LC), stationary phases that invoke alternative additional interactions afford method developers viable options for improving retention and selectivity. Phenyl stationary phases are an alternative that are thought to benefit from π-π mechanisms, which promote additional discriminating interactions between solute and stationary phase (1). Recently, there has been a growing interest in the use of phases based on the biphenyl moiety and the advantages the surface chemistry often exhibits in the separation of compounds not well resolved by C18 or existing phenyl phases. There are studies that have evaluated commercially available phenyl phases (2); however, there are limited data in the literature that characterizes the molecular interactions on biphenyl phases. In this work, the retention mechanisms of biphenyl phases are investigated and contrasted to those of moreâcommon alkyl phases.
Initial studies conducted to characterize the retention and selectivity on biphenyl stationary phases yielded insight into potential existing molecular interactions. These evaluations were based on methods described by Euerby and Tanaka, among others, and rely on using a set of defined probes to determine the relative extent of particular molecular interactions (3,4). For example, the hydrogen bonding capacity for a given stationary phase is determined by comparing the capacity factors of phenol versus caffeine. Phenol is a hydrogen bond donor whereas caffeine can accept hydrogen bonds. The difference in hydrogen bond donating versus accepting potential of these probes can discriminate the stationary-phase potential for this particular dipolar interaction.
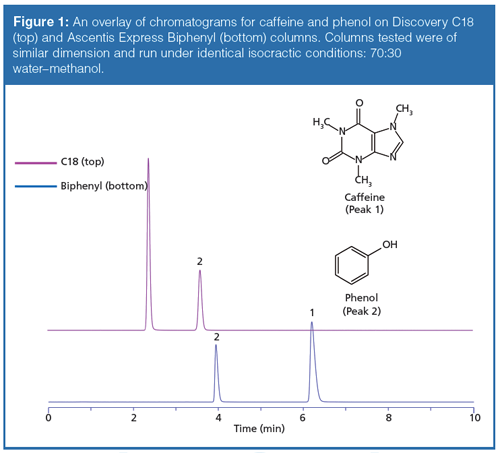
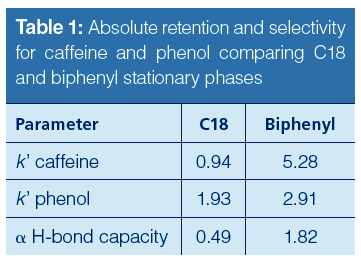
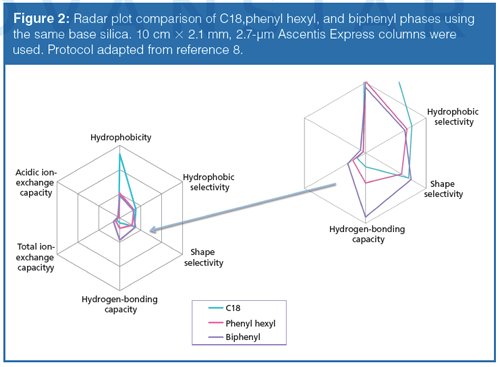
The initial studies led to the observation that hydrogen bonding capacity on the biphenyl phase was much higher compared to that of a C18 alkyl phase. Figure 1 and Table 1 describe the results of this test. An elution order reversal is observed because of the greater retention of caffeine by the biphenyl phase. Horak and colleagues (5) described a similar result. The rationale provided for the observations was that the extended π system of the purine ring compared to phenol drives retention by π-π interactions. Further verification of the trend was examined using different stationary phases bonded to the same base silica. Figure 2 again shows the relatively strong retention of caffeine by the biphenyl phase as compared to the C18 phase. Note that the phenyl hexyl phase falls between the C18 and the biphenyl in terms of relative caffeine retention. While the contributions of π-π type interactions are likely important for the aromatic stationary phases, the purpose of the current study is to further investigate the interesting hydrogenâbonding capacity observation. This was accomplished through comparison and interpretation of retention factors for a selected set of substituted benzene probes.
Experimental
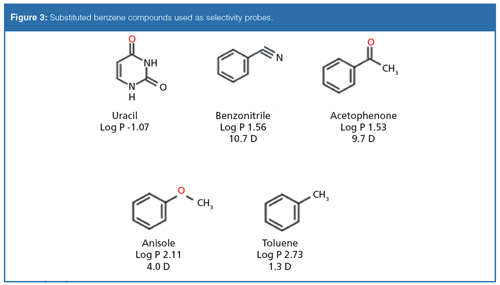
All analyses were conducted using a Dionex Ultimate 3000 HPLC system (Thermo Fisher Scientific). Ascentis Express C18, Ascentis Express Phenyl Hexyl, and Ascentis Express Biphenyl columns were obtained from MilliporeSigma/Supelco. Column dimensions were 100 mm × 2.1 mm, and they were packed with 2.7-µm solid-core particles. High performance liquid chromatography (HPLC)-grade acetonitrile, methanol, and water were obtained from MilliporeSigma. Uracil, benzonitrile, acetophenone, anisole, and toluene were obtained from MilliporeSigma. Working solutions were prepared in 85:15 water–methanol or water–acetonitrile. Column temperature was maintained at 35 °C. A flow rate of 0.5 mL/min was used and detection was via ultraviolet (UV) absorbance at 254 nm.
Results and Discussion
To understand the additional contributions to retention conferred by the functional group on the substituted benzene compounds, toluene was used as a reference. Isoelutropic conditions were selected so that the retention of toluene could be normalized for both stationary phases and organic modifiers. The biphenyl stationary phase was compared against a phenyl hexyl phase and a C18 phase to evaluate contributions to retention inherent to increasing the aromatic character of the stationary phase. The probes were chosen to include compounds that all were hydrogen-bond acceptors based on the previous observation that the biphenyl phase has a high degree of hydrogen-bonding capacity.
The results of the substituted aromatic comparison are shown in Figure 4. Changing the organic component in the mobile phase had a significant impact on selectivity. The differences in retention and selectivity are more pronounced on the phenyl phases, particularly the biphenyl stationary phase. The change in elution order between benzonitrile and acetophenone is observed on all phases, implying the selectivity difference is likely not an attribute of stationaryâphase aromaticity alone. In the methanolâbased mobile-phase solute, retention increases with the increasing aromatic character of the stationary phase. Although there are different substituent effects on the phenyl ring based on the moieties present, there is not a clear trend between the electron density of the ring and interactions with the stationary phase. Compounds with both electron donating and electron withdrawing groups all exhibit an increase in retention using the methanol mobile phase.
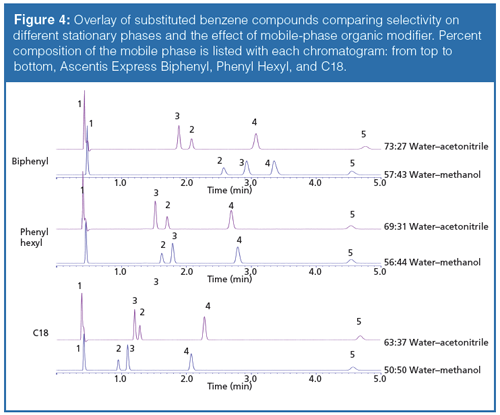
This effect of organic modifier has been observed on phenyl phases in previous studies (5). In the present study, this result is confirmed on a phenyl hexyl phase, though it is shown to be of greater significance on the biphenyl column. The accepted explanation for this observation is that π-π interactions are shielded in an acetonitrile reversed-phase environment because of the potential for charge transfer between the π electrons of the solvent and stationary phase. Although π-π molecular interactions are likely to occur, it is unclear whether this is the entire story. The explanation is likely more complex; a combination of molecular contributions and solvation dependent on organic mobile-phase component that is not yet fully understood.
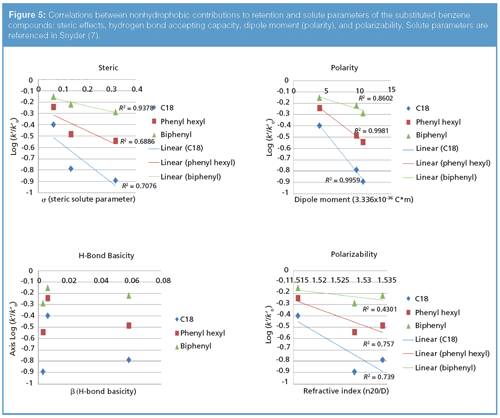
In an attempt to more fully interpret the chromatographic data for the substituted benzene compounds, a method was adapted based on the Hammet linear free energy approach (6). Using toluene as a reference compound, the (assumed) hydrophobic contributions to selectivity were subtracted so that correlations could be made between remaining molecular contributions to retention from the stationary phase. Linear regressions were then used to correlate retention to known solute parameters (7). Molecular descriptors were chosen that are most applicable to interactions for phenyl stationary phases, namely polarity, polarizability, hydrogen bond basicity, and steric contributions (shape selectivity).
The analysis of the data in Figure 5 shows no correlation between hydrogen-bond basicity of the analytes and retention on the biphenyl stationary phase, in contradiction to our previous observation in the Euerby column characterization. The most meaningful correlation was to the dipole moment, with less significant contributions from shape selectivity and polarizability. The aromatic ring, which has net negative charge above and below the plane of the ring, appears to be the major driving force in retention. Whether this is an indication of π-π, charge transfer, or polarity dictating a difference in the stationary solvation remains unclear. The data clearly demonstrate that parameters like shape selectivity, polarizability, and polarity of the analyte should be parameters that can be exploited for successful method development. These factors appear to be the major contributors to selectivity that will ultimately promote resolution of critical pairs in an analysis. Given the small sample set of probes and the potentially limited span of molecular interactions for this group of analytes, a more in-depth linear solvation energy relationship (LSER) study
is likely needed.
Conclusions
The biphenyl stationary phase exhibits an enhancement of many of the effects associated with phenyl stationary phases. Significant differences in selectivity based on the choice of organic modifier distinguish it from alkyl phases. Factors such as polarity, shape selectivity, and polarizability are indicated as the driving forces behind alternative retention and selectivity, though the relative contributions of these interactions are still unknown. The differences compared to alkyl and even existing phenyl chemistries make this phase a suitable tool for method development in reversedâphase LC where existing approaches lack efficacy. There also appears to be a need for an in-depth study of biphenyl phases to better understand and exploit the unique blend of interactions afforded by the surface chemistry.
References
- R.R. Brindle and K. Albert, J. Chromatogr. A757, 3 (1997).
- D.H. Marchand, K. Coes, J.W. Dolan, L.R. Snyder, R.A. Henry, K.M.R. Kallury, S. Waite, and P. Carr, J. Chromatogr. A1062, 65–78 (2005).
- M.R. Euerby, P. Petersson, W. Campbell, and W. Roe, J. Chromatogr. A1154, 138–151 (2007).
- N. Tanaka, Y. Okuda, K. Iwaguchi, and M. Araki, J. Chromatogr. A239, 761 (1982).
- J. Horak, N.M. Maier, and W. Linder, J. Chromatogr. A1045, 43–58 (2004).
- L.P. Hammet, Physical Organic Chemistry, (McGraw-Hill, New York, London, 1940), chapter 7.
- L.R. Snyder, J.W. Dolan, and P.W. Carr, J. Chromatogr. A1060, 77–116 (2004).
- M.R. Euerby and P. Petersson, J. Chromatogr. A994, 13–36 (2003).
Daniel Shollenberger is a Senior Research Chemist at Arkema in the Analytical Research and Services group. He has a Master’s of Science in chemistry from Lehigh, where he focused on analytical techniques in the pharmaceutical sciences. He spent the majority of his career with Supelco, studying and developing LC and GC stationary phases and sample preparation devices. In his current role with Arkema, Dan supports development of new chemicals and polymers through analytical separations, chromatographic method development, and mass spectral analysis.
Hugh Cramer received an associated degree in business management from Penn State. He began working at Supelco (now MilliporeSigma) in 1986 in GC capillary QC. Cramer later supervised the QC area for several years before joining the application area in LC R&D where he has worked for the past 13 years.
David S. Bell is R&D manager of Separations Technologies at MilliporeSigma (formerly SigmaâAldrich/Supelco). With a B.S. degree from SUNY Plattsburgh and a Ph.D. in analytical chemistry from The Pennsylvania State University, Dave spent the first decade of his career within the pharmaceutical industry performing analytical method development using various forms of chromatography and electrophoresis. During the past 20 years, working directly in the chromatography industry, Dave has focused his efforts on the design, development, and application of stationary phases for use in HPLC and hyphenated techniques. In his current role at MilliporeSigma, Dr. Bell’s main focus has been to research, publish, and present on the topic of molecular interactions that contribute to retention and selectivity in an array of chromatographic processes. Direct correspondence to: LCGCedit@ubm.com

Evaluating Body Odor Sampling Phases Prior to Analysis
April 23rd 2025Researchers leveraged the advantages of thermodesorption, followed by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS), to compare and assess a variety of sampling phases for body odor.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)