Turning Up the Heat – The Effect of Temperature on Analytical Extractions
Temperature impacts solubility, diffusion, surface tension, and more. It can potentially lead to solute decomposition. This month we will explore the role of temperature in analytical extractions.
The application of temperature is frequently employed to enhance analytical extractions, especially with solid samples or volatile analytes. Dating back to the earliest development of modern chemical analysis, such as with Soxhlet extraction, using temperature to enhance analytical extractions continues today with more recently developed techniques. Too often, chemists rely on the rule of thumb from chemical kinetics, which states that reaction rates double for every 10 degree temperature change. However, extractions are not reactions. Temperature impacts solubility, diffusion, surface tension, and other properties of the sample, potentially leading to solute decomposition. This month, we explore the role of temperature in analytical extractions.
Who am I to disagree with Bill Gates? Like the Microsoft founder, I found the book Range: How Generalists Triumph in a Specialized World by David Epstein (1) to be one of my recommended, thought-provoking reads during the COVID-19 pandemic. Epstein argues that, with exceptions like golf which require rote repetition (such as muscle memory), a breadth of knowledge and experiences are essential for personal and professional development. He claims that innovators and “systems thinkers” cultivate an “ability to connect disparate pieces of information from many different sources” (1). This philosophy is embraced in the American Chemical Society’s push to include systems thinking of addressing the United Nations Sustainable Development Goals (see, for example, a special issue of Journal of Chemical Education [2]).
When it comes to analytical problem solving, the hyperspecialization of our discipline into high-resolution mass spectrometrists, vibrational spectroscopists, open-tubular chromatography gurus, and others can be to our detriment, because the analyst must understand what the nature is of the problem at hand, how might the samples of interest be similar to or different from previous samples, and what tools are available in the analytical arsenal to solve the problem. Fundamental knowledge of our techniques, and their limitations, is essential to applying them in the most appropriate manner.
Many of our analytical extraction methods manipulate temperature to enhance extraction yields. In Soxhlet extraction and its derivatives, accelerated solvent extraction (ASE), also called pressurized solvent extraction (PSE), microwave-assisted extraction (MAE), ultrasound-assisted extraction (USAE), and related methods all use increased operating temperatures to improve extraction yields and accelerate the rate of extraction. As a consequence of the increased solute solubility, solvent use can be dramatically decreased compared to room temperature methods. For volatile analytes, temperature is often elevated with purge and trap, static or dynamic headspace, or thermal desorption systems to drive compounds of interest into the gas phase for trapping and analysis. However, whenever increased temperature is applied to an analytical extraction, it is not as simple as turning up the heat, and more heat (higher temperatures) is not necessarily better. Thermal degradation of sample components is always a concern, but it is also important to note that, while there is a kinetic effect to elevated temperatures, it is thermodynamics that drives the extraction in many cases. In subsequent sections, we provide a broad overview of these temperature effects on analytical extractions.
Solubility and Partitioning
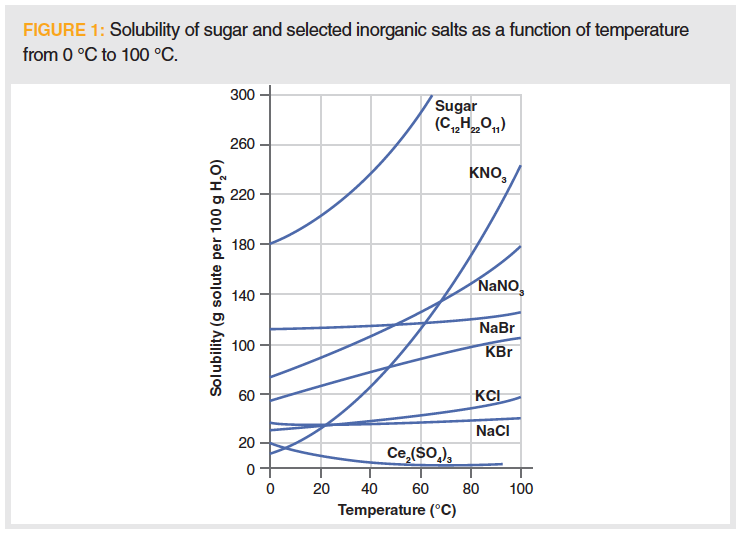
Generally speaking, increasing temperature increases the solubility of solid solutes in liquid solvents. However, the extent to which this occurs may vary dramatically between solutes. For example, we can see in Figure 1 that the solubility of sugar and potassium nitrate in water changes dramatically as a function of temperature. For sugar, the aqueous solubility of sugar approximately doubles with a temperature increase of 40 °C, and the effect is even greater for potassium nitrate. On the other hand, the water solubility of salt (sodium chloride) is essentially unchanged over a broad temperature range, while for cesium (III) sulfate (Ce2[SO4]3), there is even a solubility decrease as the system rises above room temperature. Now imagine the most probable scenario when our system of analytical interest is a mixture. We cannot be assured extraction enhancement, because increased solubility as a function of temperature is not equivalent for all analytes of interest. Luckily, it is exceedingly rare where we work close to the solubility limits of a chemical system.
A special case of applying increased temperature to liquid solvents is with so-called subcritical or superheated water. At temperatures above 200 °C up to the critical temperature (374 °C), the dielectric constant of water is lowered until it is similar to some common organic solvents (note that increased pressure is also applied to keep water in its liquid state). In this case, the added thermal energy is sufficient to begin to disrupt the hydrogen bonding between water molecules, allowing the “hot water” to begin to solubilize nonpolar solutes, which may be completely insoluble in conventional liquid water. This is the basis for “hot water extraction,” which has shown some promise in the literature, but has yet to be exploited for widespread use.
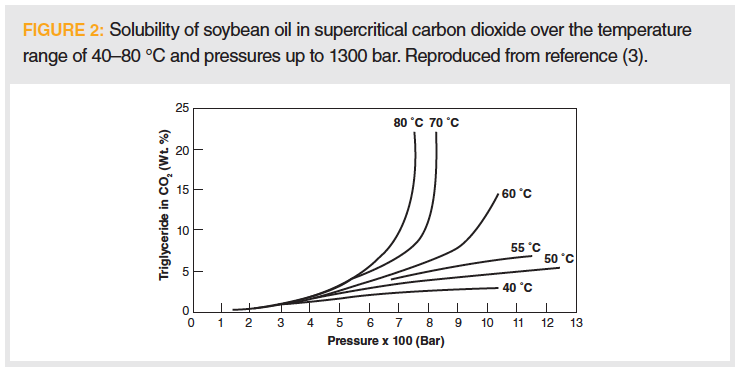
In supercritical fluid extraction (SFE), the situation is even more complicated. The solvating power of a supercritical fluid is directly related to the fluid density, which is a function of both temperature and pressure. Within a given extraction, pressure is most easily controlled, so temperature is held at a selected value and pressure is used to adjust density (change solubility) if selectivity during extraction is desired. At constant pressure (often the case during an analytical extraction), increasing temperature will decrease density, and, hence, solubility. However, this is not always the case, and, when it is, there is not a clear relationship between temperature, pressure, and solubility. For example, Figure 2 shows the solubility of soybean oil in supercritical carbon dioxide. In this diagram, solubility of the triglycerides is fairly low until a certain temperature (60–70 °C in this case) is reached, at which point solubility increases to a great extent. We can infer that solute fugacity may play some role and numerous equations of state have been developed to predict density and corresponding solubility. Adding an additional level of complexity, and not addressed in this column, is the common approach of adding small amounts (5–20%) of organic co-solvent (modifier) to increase solute solubility in supercritical fluids.
We often use equilibria to describe analytical extractions, though, in many (if not most) cases, we do not operate at equilibrium conditions. Commonly, the octanol-water partition coefficient, Kow, is used to estimate relative solubility in extraction solvents:
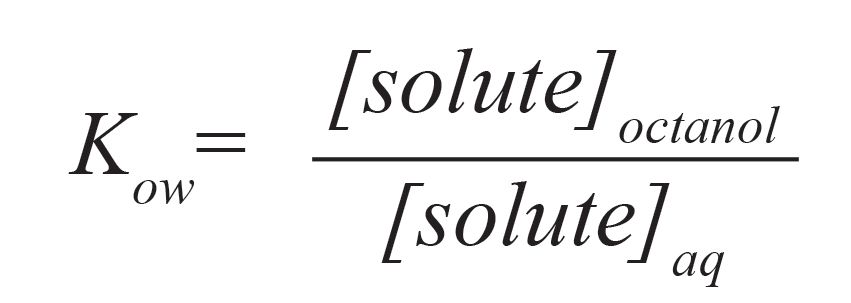
as the octanol solubility may, for example, mimic solute partitioning through a nonpolar lipid cellular membrane. Given that solute solubility as a function of temperature varies greatly from solute to solute, can we predict what may happen to Kow values? While there are no simple generalizations, we can look at the heat of solution and apply Le Chatelier’s principle—that is, if the dissolution process is exothermic (releases heat), the partition coefficient should decrease with increasing temperature, while for endothermic systems, which absorb heat, the corresponding partition coefficients will increase. The magnitude of the change in partition coefficient will be proportional to the magnitude of the change in the molar heat of solution.
Volatility
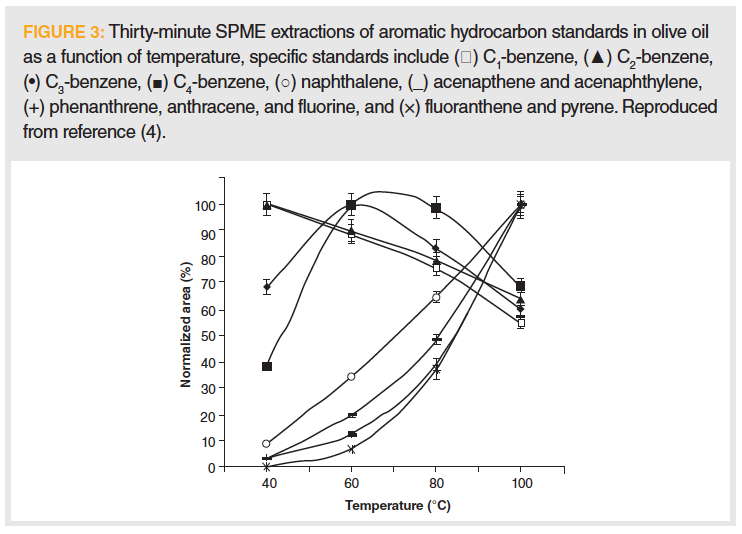
Like solubility, relative volatility can vary to a large extent with temperature, and is dependent on operating pressure and the contribution of other components of the sample mixture. This has even led some to claim that headspace extractions may not be quantitative and was the subject of this “Sample Prep Perspectives” column about two years ago (4). In that column, we claimed, “It makes sense that heating will increase the rate of evaporation, enhancing the mass transfer, and driving analytes into the vapor phase, thus increasing their concentration for subsequent collection. The problem lies in the fact that different molecules possess different volatilities and, thus, differing amounts extracted” (4). This is demonstrated in Figure 3, where aromatic hydrocarbons are sampled using solid-phase microextraction (SPME) in the headspace above samples of olive oil. The results show that, as the sample is heated, the behavior of different sets of compounds change in a less than straightforward manner. This resulting change in headspace composition as a function of temperature not only influences the extraction yield of an individual analytes, but it could also potentially be exploited to gain selectivity or the ability to isolate one com- pound in preference to others in the sample. As a result, we admonished that the extraction temperature during headspace analysis must be well-documented.
Thermodynamic Parameters
It is well-established that chemical extractions do not occur solely via solubility concerns, but also due to the system thermodynamics. Most important, especially when isolating analytes from solid samples, is the role of solute diffusion through the solid. Diffusion is often described using the Stokes-Einstein equation, D = kT/6πηa, where D is diffusivity, k is a proportionality constant, T is temperature, η is viscosity, and a is activity. At first glance, there is a direct propor- tionality between diffusion and temperature, which means higher temperatures result in fast diffusion. Diffusion, though, is also a characteristic of other solvent properties like viscosity and surface temperature. Starting with viscosity, we can describe the temperature dependence with the Andrade equation, μ = AeB/T, where dynamic viscosity is related to temperature (T) through an exponential function and the constants A and B are available in comprehensive thermodynamic tables. The relationship between surface tension and temperature is an even more complex function also involving the solvent critical temperature as surface tension decreases to zero at the critical temperature. Thus, increasing temperature will increase solute diffusion, speeding extractions, but the relationship with extraction rate is not necessarily linear. We have previously discussed that the effect of diffusion is independent of extraction type (ASE/PSE, MAE, USAE) at constant temperature (6).
The case of supercritical fluids is a bit more confounding. Solute diffusion in supercritical fluids is indirectly related to density via the fluid viscosity. The diffusivity in these fluids decreases with increasing pressure at constant temperature and increases with increasing temperature at constant pressure.
Kinetics or Rates of Extraction
Extractions generally follow a first-order rate model, similar to the kinetics of chemical reactions. However, extractions are due to a combination of solubility and analyte diffusion. Neither alone dictates speed of a reaction and, as we have seen, neither is necessarily a linear function of temperature. So, while adages like “doubling the rate with every ten degree temperature change” may have merit to estimate reaction kinetics, this does not hold for analytical extractions.
Sample Thermal Degradation
Any time we heat a sample, breakdown, usually by oxidation, is a concern. A good analytical practice is to run suitable standards or well-characterized samples and check for degradation products. Is the degradation concern exacerbated with the temperatures used in modern analytical extractions? This was a concern in the early development of ASE and was addressed in research by Ezzell (7). He looked at the ASE of dicumyl peroxide (DCP), a known free radical generator. Half-life calculations predicted that up to 30% decomposition would be expected in at 7.5 min extraction at 150 °C. Next, DCP was spiked onto sand and extracted via ASE with hexane, first at 100 °C, then at 150 °C, with a 10 min heat and extract time. At 100 °C, no sample degradation was observed, as 101% recovery with a 5.9% relative standard deviation (RSD) was obtained. With an extraction at 150 °C, extraction recovery dropped to 77%, close (within 75–80%) to the estimated 30% decomposition. In both cases, the solvent bottle was pressurized with air and was not degassed. When the hexane was degassed and pressurized with nitrogen, the extraction yield at 150 °C increased to 91% with a 0.83% RSD, demonstrating that when proper precautions are taken, thermal decomposition can be avoided in many cases. It is assumed that these findings, with similar precautions, are transferrable to other techniques. For example, Ezzell also noted that degradation of unsaturated fatty acids during extraction of triglycerides from oilseeds with ASE at 130 °C was similar to that observed during Soxhlet extraction. However, free-radical generation with ultrasound extractions is well-known among practitioners, in this case because of the high sonic energy applied during the extraction.
Conclusions
The development of modern analytical extractions has progressed through the application of increased extraction temperature. In fact, aside from solute solubility in the chosen extracting solvent, applied temperature is perhaps the single most important parameter (along with particle size, for solid samples) driving sample extractability and, to a lesser extent, selectivity. However, to most effectively exploit the role of temperature in extraction, the analyst must have the suitable combination of knowledge and experience with their sample type and their extraction techniques.
References
- D. Epstein, Range: How Generalists Triumph in a World of Specialists (Riverhead Books/Random House, New York, New York, 2019).
- P.G. Mahaffy, F.M. Ho, J.A. Haack, and E.J. Brush, J. Chem. Educ. 96, 2679–3044 (2019).
- J.W. King, Grasa y Aceites 53, 8–21 (2002).
- D.E. Raynie, LCGC North Am. 37(1), 10–14 (2019).
- S. Vichi, L. Pizzale, L.S. Conte, S. Buxaderas, and E. Lopez-Tamames, J. Chromatogr. A 1090, 146–154 (2005).
- D.E. Raynie and R.E. Majors, LCGC North Am. 32(6), 396–403 (2014).
- J. Ezzell, Thermo-Fisher Scientific Technical Note 206 (2014).

“Sample Prep Perspectives” editor Douglas E. Raynie is a Department Head and Associate Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high-resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee. Raynie is a member of LCGC’s editorial advisory board. Direct correspondence about this column via e-mail to amatheson@mjhlifesciences.com
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.





