Untargeted LC–MS Lipidomics with Data Independent Acquisition using Sequential Window Acquisition of All Theoretical Fragment-Ion Spectra
A focus on untargeted UHPLC–MS/MS lipidomics with data independent acquisition using SWATH technology. This article explores the wide-ranging potential and peculiarities of SWATH–MS hyphenated with UHPLC for the profiling of lipidomes of various biological samples.
Image Credit: Mateusz/stock.adobe.com
Lipidomics is a subdiscipline of metabolomics yet has established itself as an independent “-omics” sector for better coverage of lipids. A number of different workflows are routinely used, each of which has its advantages and drawbacks. This article will highlight the benefits of untargeted UHPLC–MS/MS lipidomics with data independent acquisition (DIA) using sequential window acquisition of all theoretical fragment-ion spectra (SWATH).

Lipidomics approaches are now widely adopted to obtain a comprehensive view on lipid profiles in biological samples (1). Alterations in lipid profiles may provide a panel of biomarkers for diagnostic and prognostic purposes of various diseases and yield a useful basis for the interpretation of biochemical processes. Untargeted lipidomics workflows may generate new hypotheses for supporting biological interpretations or complementing other “-omics” data. Targeted lipidomics approaches are often used to validate biomarkers found in a discovery phase or measure particular lipids associated with a certain disease or present in low abundance (for example, lipid mediators in inflammation [2]). Considering the importance of many lipids in signalling pathways and membrane integrity, lipidomics has gained wide interest not only amongst analytical chemists but also in the clinical research community.
From an analytical perspective, the workflow for comprehensive analysis of lipids is not as streamlined as it is in proteomics. A variety of different strategies are now employed, each one with its strengths and weaknesses. Shotgun lipidomics (3) by direct infusion of the lipid extract from a biological system into a high‑resolution mass spectrometer (MS) such as Fourier-transform ion cyclotron resonance mass spectrometry (FTICR–MS) or orbital ion trap MS is fast and has high‑throughput capabilities but may suffer from ion suppression, in-source fragmentation, and assay specificity problems (especially with instruments having lower mass resolving power such as quadrupole time‑of‑flight mass spectrometry [QTOF-MS]). Lipids have many isomers and a chromatographic separation before MS detection may therefore resolve some assay specificity issues. Under this perspective, a variety of approaches have been proposed using either ultrahigh‑performance liquid chromatography (UHPLC) or supercritical fluid chromatography (SFC) as separation techniques. Normal‑phase liquid chromatography (NPLC), hydrophilic interaction chromatography (HILIC), and SFC yield lipid class separation (with coelution of lipid species with distinct side chains or rather elution in a narrow window) (4), while reversed‑phase liquid chromatography (RPLC) affords lipid species separation with some overlaps between lipid classes (5,6). Gas chromatography–mass spectrometry (GC–MS) has its domain mainly in full comprehensive fatty acid (FA) profiling as fatty acid methyl esters (FAMEs) (7). In this article we intend to shed some light on ultrahigh-pressure liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) using QTOF instruments and data independent acquisition (DIA) with sequential window acquisition of all theoretical fragment-ion mass spectra (SWATH) (8).
Common Acquisition Modes in LC–MS Lipidomics
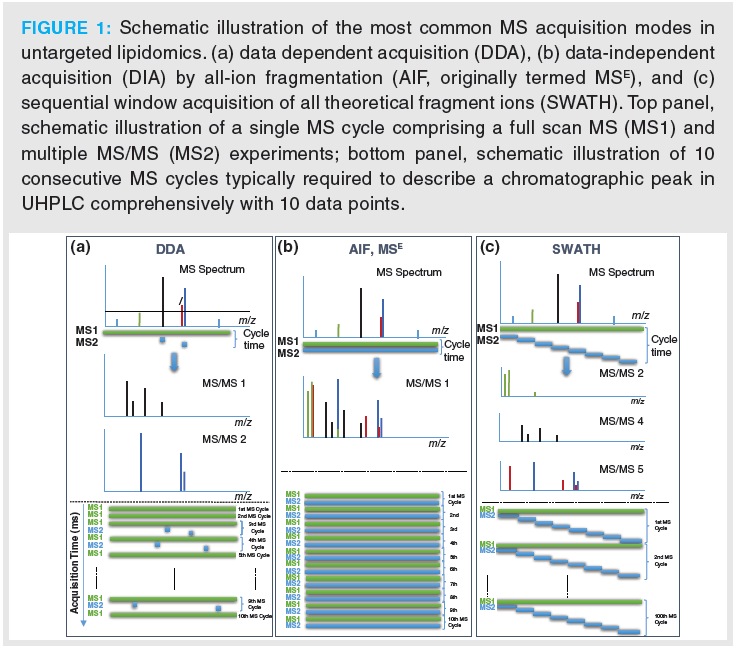
Lipidomics can be performed with or without chromatographic separation, with or without integrated ion‑mobility using a differential mobility spectrometry (DMS)‑driven lipid class separation concept without liquid chromatography (LC) (9) or with/without LC using travelling wave ion‑mobility (TWIM) (10), drift‑tube ion‑mobility mass spectrometry (DTIMS‑MS) (11), or trapped ion mobility spectrometry (TIMS) with TOF‑MS (12), and with different MS instruments as well as distinct acquisition modes (Figure 1 and Table 1).
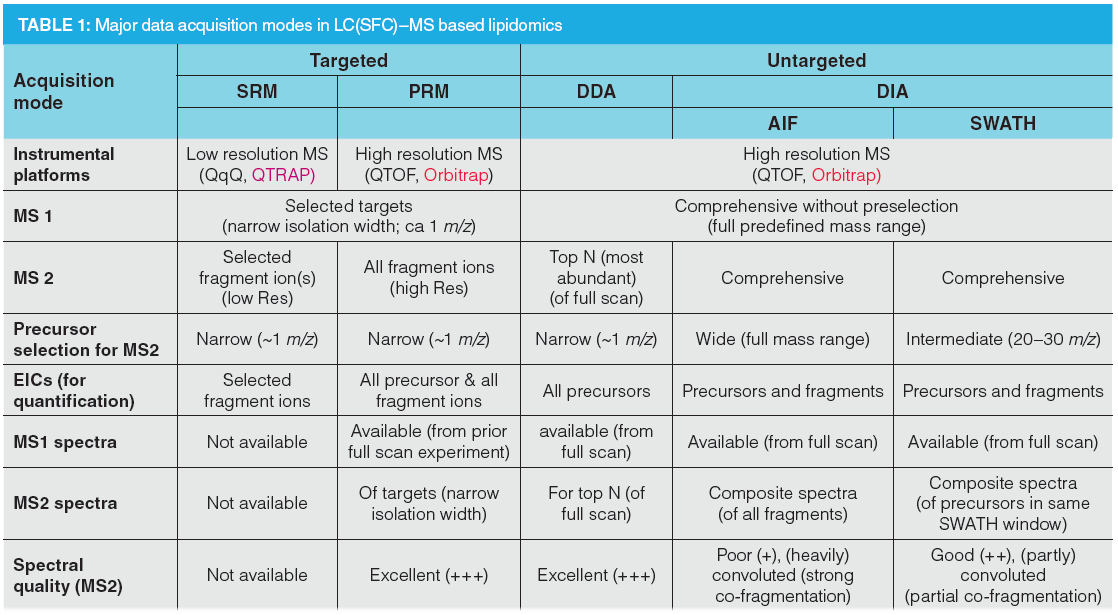
Targeted lipidomics is based on MS instruments with low mass resolving power such as triple quadruple (QqQ) or quadrupole-linear ion trap (QLIT) using selected reaction monitoring (SRM) data acquisition (13) or high‑resolution MS (HRMS), such as QTOF or orbital ion trap MS in parallel reaction monitoring (PRM) mode (also termed “high resolution MRM”) (14). For both, the targets have to be preselected and programmed before acquisition: in SRM both precursor and fragment ions (therefore only selected fragments measured in MS2), for PRM only the precursor ion (all fragments of targets acquired). For this reason, the former requires more method development efforts. If targeted lipidomics is performed in a scheduled mode, that is, respective ion transitions are acquired only during the time when the respective compounds elute, today’s modern and highly sensitive instruments allow measurement of a similar number of lipids as with untargeted approaches (in particular if we consider for the latter only the compounds that are finally identified with sufficient confidence). Lipid coverage for targeted triple quadrupole assays is typically in the several 100s to up to the 1000s. Targeted assays acquired with triple quadrupole in SRM benefit from their robustness, wide dynamic range, sensitivity, and good quantification performance. However, the targets need to be programmed in advance and molecular parameters which are not on the target list during acquisition are missed. In case of PRM, unexpected findings may be still covered at the MS1 level by acquiring a full scan experiment prior to product ion scans of the targeted analytes (14).
However, more popular in lipidomics research are untargeted assays. The state-of-art in UHPLC–MS/MS lipidomics is data‑dependent acquisition (DDA) using orbital ion trap MS (with higher mass resolving power) or QTOF instruments (with faster acquisition rates) (Figure 1[a]). DDA is based on an MS1 full scan (over a certain predefined mass range) and uses information from this full scan experiment to select a small number of precursors (top N), for example in a top four experiment the four most abundant precursors, for fragmentation. High quality MS2 spectra linked to their precursors are obtained. However, coverage is incomplete and precursor selection is a stochastic process that lacks reproducibility between samples because it depends on the actual conditions, frequently leading to missed triggering of MS2 spectra. Since MS2 data are not collected continuously, extracted ion chromatograms (EICs) can be extracted only on MS1 level; hence, quantitation is restricted to the MS1 level. It is therefore not possible to support quantitative results by control via a qualifier ion. Thus, limited MS2 coverage restricts identification performance and lack of fragment ion EICs limits the quantification performance of DDA.
DIA came into the focus of MS‑based lipidomics technologies (8) after it had demonstrated its potential in proteomics (15). It is performed on high resolution instruments, including QTOF-MS, orbital ion trap-MS and can overcome some of the above limitations mentioned for DDA. DIA is a good strategy for acquiring comprehensive MS and MS/MS data over an entire range of m/z values: all precursor ions are fragmented. Thus, most distinctively from DDA, DIA collects MS2 data comprehensively over the entire chromatogram and across all samples without dependence on information from the prior full scan (MS1) experiment. The result is a comprehensive digital map (MS1 and MS2 data) of the lipid profile of a sample. Therefore, retrospective data analysis can be repeated at later stage without restrictions once new databases with more lipid entries become available or new algorithms for identification of lipids have been developed without need for re‑measuring samples that are then often not available anymore. It is therefore ideal for data (bio)banking such as required in personalized medicine.
A number of DIA varieties exist which essentially differ in their precursor isolation width: In all-ion‑fragmentation (AIF or MSE) (Figure 1[b]), subsequent to a full scan MS experiment (MS1) over a certain mass range an MS/MS experiment is carried out in which the MS1 (quadrupole) is tuned to all ion transmission (like in full scan) and the collision energy (CE) is elevated as compared to the MS1 experiment. In other words, two full scan experiments are performed, one with low CE and one with high CE that imparts fragmentation to the precursor ions. The precursor isolation width is wide (full m/z range) which leads to co‑fragmentation of all isolated precursors simultaneously. In SWATH acquisition (Figure 1[c]), subsequent to an MS1 full scan a series of MS/MS experiments with consecutive, (usually 1 m/z)-overlapping intermediate-sized precursor isolation (SWATH) windows is carried out which cover the entire mass range of interest. The SWATH windows can be either of fixed width, such as 20–30 m/z or of variable width (width of SWATH windows adjusted that each window has either a constant ion population or a constant ion current, optimized with software) (16). When designing the SWATH experiments, it is also important that there are enough data points across the chromatographic peak (at least 10) to enable robust and precise quantification. If the chromatographic peak has about 6 s, which is a common peak width of UHPLC, then the entire MS cycle (comprising both MS1 and all MS2 acquisition times together) should not take longer than 600 ms. It means that the design of the SWATH windows turns out to become a compromise between narrow (precursor isolation) SWATH windows (preferred in terms of assay selectivity) and long accumulation times (advantageous with regard to sensitivity). Typically, 20–30 SWATH windows with accumulation times of 20–30 ms are programmed per MS cycle. Variable SWATH windows and other smart designs with particular focus on analytical assay requirements (regarding selectivity and sensitivity for specific analytes) become possible and improve the assay performance. For instance, in a combined targeted/untargeted steroidomics assay specific SWATH window sizes of 4 m/z width for estradiol (E) and testosterone (T) (instead of the common 20–30 u wide windows) were established whereby the 13C3‑labelled surrogate calibrants were recorded in the same SWATH window as the corresponding analyte and the d5-analogues that were used as internal standards in a separate 5 m/z wide SWATH window (to avoid interferences) (17). Accumulation times could be individually adjusted for each SWATH window to reach the required detection limits (300 ms for E and 13C3‑E as well as 100 ms for d5E; 50 ms for T, 13C3‑T and d5T). Around these targets additional SWATH windows were constructed to allow for simultaneous qualitative and quantitative analysis. In a platelet lipidomics study, a variable SWATH window approach was selected with 5 m/z (u) target windows for oxidized phospholipids which were measured as markers of oxidative stress embedded in a SWATH design in which the window size was optimized with swathTUNER to obtain a constant precursor ion density in each SWATH window (18). A hybrid design with HR–SRM/MS using multiple unit mass windows and SWATH/MS with 100 u precursor selection windows has been proposed by Hopfgartner and co-workers for phospholipidomics using HILIC for lipid class separation (19). Since individual molecular lipid species coelute in lipid class separation, the unit isolation width guarantees that isomeric lipids can be distinguished on MS2 level without isotopic interferences from other lipids.
Cycle times are shorter in AIF than in SWATH enabling longer accumulation of ion currents leading to enhanced sensitivity. A major drawback of DIA for identification is that the MS2 spectra cannot be linked to their precursors. In SWATH, they can be assigned to the specific mass range of the SWATH window, while absolutely no assignment is possible in AIF. This has led to the development of another DIA mode: scanning quadrupole DIA (20) or scanning SWATH (21). It collects MS2 data while scanning in Q1 over a certain m/z range; the advantage of this acquisition mode is that fragment ions can be assigned to the precursor because of the timewise link of MS1 and MS2, or in other words the Q1 position at which certain MS2 fragment ions are recorded, is known. However, this acquisition is not very popular yet.
These data acquisition modes by DIA come with significant advantages, but also some disadvantages. Since there is no dependence on information by the survey scan, MS2 data offer better reproducibility, which is beneficial for identification reproducibility. MS2 data are available for all precursors and hence analyte coverage is usually better. The downside with regard to identification is the loss of the link between precursor and fragment ions (as mentioned above) and poorer quality of MS2 spectra due to co‑fragmentation (which could be overcome by scanning SWATH). Coeluting analytes which are co‑isolated in the same precursor isolation window for fragmentation are co‑fragmented in the collision cell and hence composite spectra are resulting. As a result of wider precursor isolation windows than in DDA, fragment ions in DIA feature also isotopologue peaks (unlike to DDA) which may be helpful for identification. The real advancement of DIA, in turn, is related to its better quantification performance. EICs can be generated on MS1 for precursors and on MS2 level for both precursors and all fragment ions. It allows post‑acquisition selection of the most suitable EIC for quantification, either most selective or most sensitive ion, and at the same time intra‑assay validation, that is, confirmation of results by control via other EICs. In other words, it means that in addition to the quantifier ion, a number of qualifier ions are also available for compounds with reasonable fragmentation. From this viewpoint, reliability of DIA assays may be better than for DDA ones in which quantification can be performed solely on the precursor ion at MS1 level. The flexibility of SWATH designs has already been pointed out above; it should be added here that certain MS parameters can be individually adjusted for each SWATH window. For example, if a certain target ion is of particular interest or needs more sensitivity, the window width can be narrowed to gain assay specificity and accumulation time can be elevated and/or CE optimized to enhance sensitivity of this target. A point of particular interest in clinical research is that SWATH allows uncompromised retrospective data processing (because all MS2 data are also available) which is particularly useful for data repositories in the context of personalized medicine. Its utility in lipidomics to find diagnostic, prognostic, and monitoring biomarkers, to allow for validation of hypotheses and derive new ones is of great interest in clinical research.
Performance Characteristics of SWATH–MS in Lipidomics
The fact that MS1 and MS2 data are available comprehensively over the entire chromatogram allows optimal simultaneous qualitative as well as quantitative analysis and makes LC–MS by SWATH an ideal choice for untargeted lipidomics. Some of the performance characteristics should be illustrated here by examples.
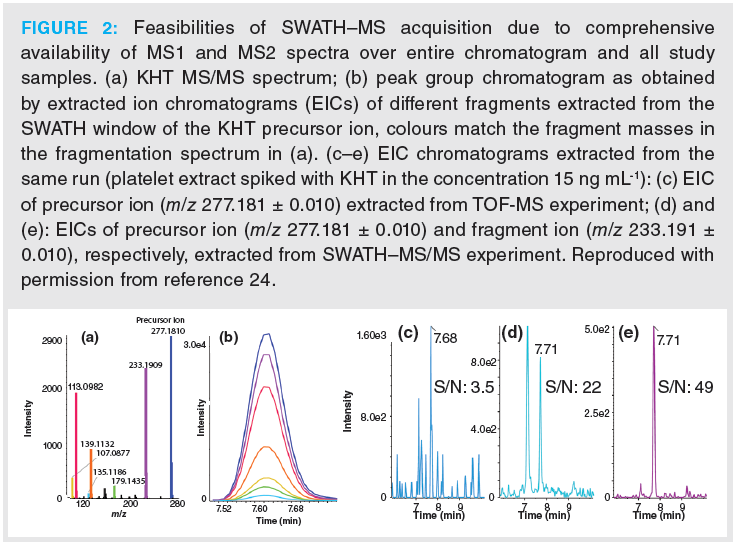
For untargeted lipidomics, we typically use a lipid species‑selective RPLC based on sub‑2‑µm fully porous particles as primary LC method (5,6,22–24). It has the benefit that it provides selectivity for many isomeric lipid species. Nevertheless, due to sample complexity selective ion traces are needed for interference free quantitative analysis. This is exemplified in Figure 2 based on the RPLC–MS/MS analysis of (oxidized) fatty acids with SWATH acquisition of a lipid extract from a human platelet sample (24). A variable window approach was used with narrow 5 u windows for three targets, amongst others, for 12-keto-heptadeca‑5Z,8E,10E‑trienoic acid (KHT) released upon platelet activation, while other SWATH windows were wider. In Figure 2(a), the MS/MS spectrum of KHT is shown. For each of the fragments, the EICs can also be extracted yielding a peak group (Figure 2[b]) for each lipid, and either one can be selected for quantitative analysis. In a real sample, background noise and interferences may be present which limit assay specificity and reduce detection sensitivity. Figure 2(c) shows the EIC of the KHT precursor ion with m/z 277.1809 from the TOF‑MS experiment. There is good signal intensity, but the noise level is high as well resulting in a poor S/N ratio. The S/N ratio gets significantly enhanced when the EIC of the precursor is extracted from the respective SWATH–MS/MS experiment (Figure 2[d]). A quantum leap in assay specificity and further improvement of the S/N ratio can be observed for the EIC generated by extraction of the characteristic fragment ion of KHT with m/z 233.191 from the SWATH window (Figure 2[e]).
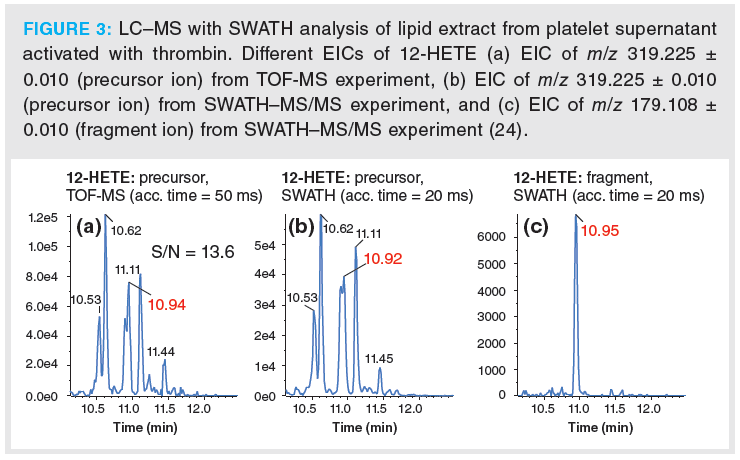
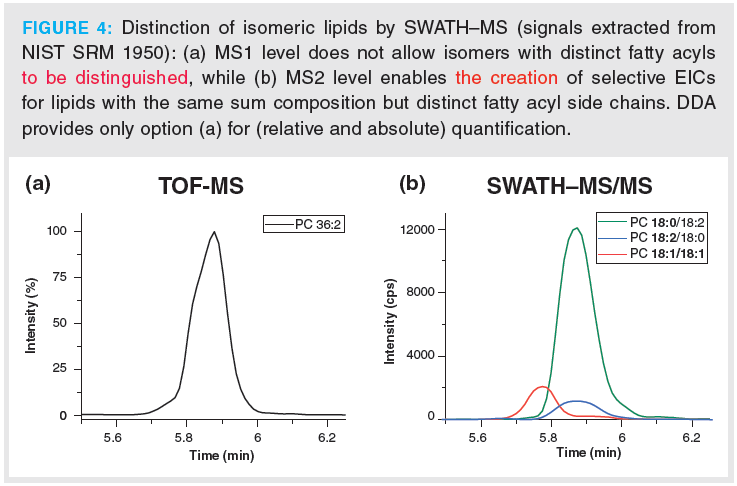
Lipids are rich in isomers which cannot be distinguished on MS1 level. On the contrary, selective EICs from MS2 can often provide adequate assay specificity. This is illustrated by chromatograms of 12-HETE in a lipid extract from human platelets (Figure 3) (24). Various positional isomers of HETE can be formed by oxidation of arachidonic acid. Hence, EICs of the precursor ion of 12‑HETE both in TOF‑MS (MS1; Figure 3[a]) as well as SWATH–MS/MS (MS2; Figure 3[b]) do not provide adequate assay specificity. In sharp contrast, the EIC of the characteristic fragment ion of 12‑HETE allows interference‑free quantitative analysis of this important biomarker of platelet activation from the SWATH–MS/MS experiment (Figure 3[c]). A similar problem exists for glycerophospholipid isomers. DDA cannot individually quantify phospholipid isomers of the same sum composition but distinct fatty acyl side chains if they are not fully resolved chromatographically. Chromatographic run times must be kept short in order to satisfy high throughput requirements of clinical studies. Unfortunately, high-sample throughput comes at the expense of chromatographic resolution of such isomers. For instance, the peak for PC(36:2) in Figure 4(a) shows a shoulder in the MS1 EIC which is due to the presence of two distinct isomers (PC18:1/18:1 and PC 18:0‑18:2). On MS1 level (relative) quantification, as with DDA, cannot distinguish between these isomeric molecular lipid species and quantification remains limited to the sum composition (Figure 4[a]). This, however, may be biologically misleading if, for example, two isomeric lipids behave oppositely in terms of up and down regulation in two sample groups and the two isomeric lipids are analyzed by DDA on MS1 level. SWATH can offer MS2 level quantification of such isomeric lipids, as shown in Figure 4(b), and offers to derive the real biological trends. However, in this context it has to be pointed out that the most abundant fragments of lipids are often less specific, that is they are rather lipid class- than lipid species‑specific (for example, product ion m/z 184.07 of PCs in ESI+ mode) (23). Unfortunately, often the more characteristic fragments are of low abundance, thus associated with lower sensitivity if they are used for quantitation. The lower signal abundance of the fragment ion is partly compensated by lower noise level leading to larger response factors (22). In general, the most suitable ion for quantitative assays need to be decided on case-by-case basis (22).
SWATH–MS is perfect for combining targeted and untargeted profiling in one analytical run which maximizes the information content collected from samples with limited accessibility, such as from animal models. For example, non‑targeted dihydrotestosterone (DHT), a bioactive metabolite of T formed by the enzyme 5α‑reductase, could be monitored in the plasma samples by an assay which was targeting estradiol and testosterone (17). Since this analyte was not targeted in advance and the MS parameters not specifically optimized for this compound, it was beneficial that a suitable signal with sufficient selectivity and sensitivity could be selected post‑acquisition for EIC generation and data processing.
In several applications it could be shown that MS2-level quantification is more sensitive than MS1 level (24–26). For free fatty acids, which do not readily fragment, MS2 level quantification can benefit from a lower background noise even when the precursor ion was selected in the respective SWATH window to generate the EIC. For example, a lower limit of quantification (LOQ; at S/N=10) and lower limit of detection (LOD; at S/N=3) of 0.75 ng/L and 0.23 ng/L, respectively, was reported for FA(16:4) n-3 in Wakame (algae) extract for MS2 level quantification based on the precursor ion. On the contrary, at a concentration of 1 ng/mL FA(16:4)n-3-d5, used for this sensitivity comparison, could not be detected in the TOF-MS scan (25).
SWATH can also be used to extend the linear range in quantitative analysis. For example, it is possible to establish two distinct calibration functions based on two different EICs, one for the low concentration range with a highly abundant ion and sensitive EIC, respectively, and another one for the high concentration range based on another EIC with less abundant ion. This is not possible in DDA assays in which only a single MS1 level EIC for each lipid can be used for quantitative analysis.
Workflows with SWATH MS-Acquisition
A full lipidomics workflow consists of several steps, each of which has to be carefully optimized and standardized (Figure 5[a]). It starts with study design and sampling that depend on the biological question to be addressed, usually a solvent extraction procedure of lipids from the sample material follows (27,28) (sometimes combined with a solid‑phase extraction [SPE] step for enrichment of low abundant lipids and removal of interfering species). The type of liquid extraction depends on the sample. Usually we prefer to use a monophasic solvent extraction (incorporating protein precipitation) with 2‑propanol (29). It works well for plasma (22,30) and also cells (29), for the latter treatment by sonication may be necessary to break the cells. Recoveries are improved for polar lipids while they are still quite acceptable for apolar lipids (such as triacylglyceride and cholesteryl esters which are usually highly abundant). This extraction solvent is also more compatible with typical plastic vials commonly used for collecting clinical samples. If samples have a high salt content or for other (selectivity) reasons (for example, large volume aqueous sample), a biphasic liquid–liquid extraction using methyl tert-butylether (MTBE)/methanol (upper layer is organic phase) may be the protocol of first choice (28). An internal standard (IS) mixture containing one stable isotope-labelled IS for each lipid class of interest is added before lipid extraction to compensate for losses during sample preparation and injection. For data acquisition we employ in our workflow preferentially lipid‑species separation by RPLC and SWATH acquisition as mentioned above. As emphasized, for lipids many isomers often exist and RPLC provides adequate selectivity. On the other hand, because only one stable‑isotope labelled IS per lipid class is added, matrix effects are not optimally corrected for each lipid species which do not co‑elute with their IS. Direct infusion HR–MS and lipid‑class separation LC–MS or SFC–MS may have some advantage in this regard as a result of co‑ionization and co‑elution of lipid‑class specific ISs.
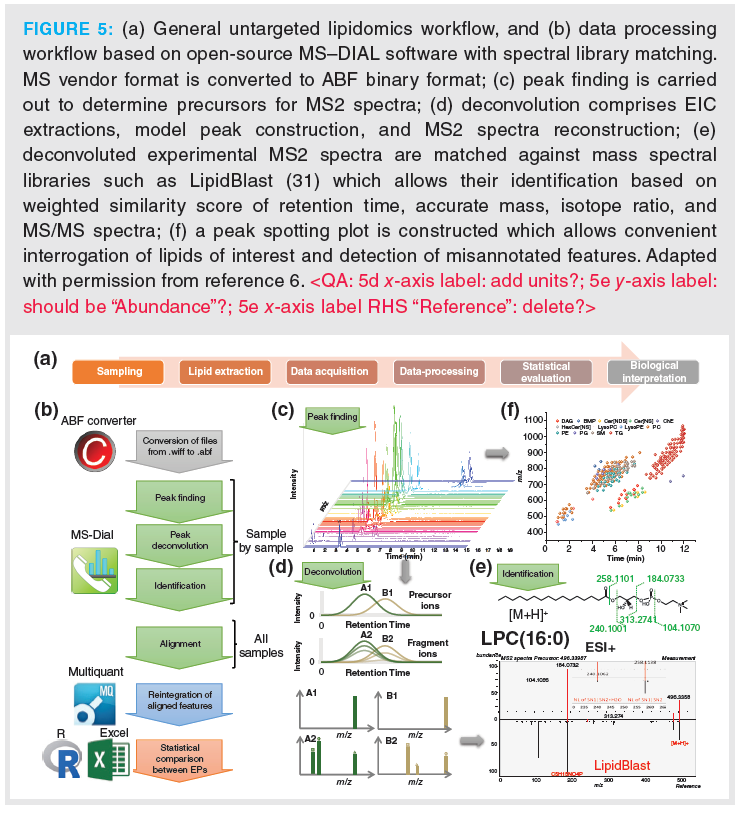
An important part of the entire lipidomics workflow is data processing. Collectively acquired data through all samples must be transformed into useful information. Limited dedicated software packages for SWATH lipidomics data are available currently and they are discussed in reference 8. Many widely used software packages for lipids and untargeted lipidomics, respectively, do not specifically support SWATH data processing. We generally employ two distinct strategies: One is based on the open‑source software MS–DIAL (6) (Figure 5[b]), the other one on targeted feature extraction of well known lipids or lipids listed in databases along with vendor software (Figure 6). MS–DIAL is a software that can process DIA data. Data processing involves the steps of peak finding, deisotoping, adduct assignment, background subtraction, feature alignment, and normalization with user‑defined settings (Figures 5[b] and 5[c]). It also offers solutions to some problematic issues of DIA, with regard to the presence of contaminating ions in the MS2 spectra of coeluted lipids which are co‑isolated in the same SWATH window for fragmentation due to similar m/z and wider precursor isolation window. As discussed above, the link between precursor and fragment ions is lost in DIA acquisition modes such as AIF and SWATH. Both problems can be resolved by a mathematical spectral deconvolution process. Briefly, from the multiplexed MS2 spectra with the contaminating fragment ions MS2 chromatograms are generated (Figure 5[d]). Peak profiles that are overlapping with the precursor ion signal are assigned to a peak group and subsequently deconvoluted MS2 spectra can be reconstructed from each peak group. The deconvoluted MS2 spectra can be matched against database spectra, such as LipidBlast (an in silico spectral library) (31) (Figure 5[e]). The structural annotation (identification) is supported by a scoring system based on retention time similarity (with calculated tR‑values from a prediction tool), MS1 (mass accuracy) similarity, isotope similarity, MS2 similarity (calculated as dot product, reversed dot product and fragment presence) (6). The process is fully automated and allows large scale lipid annotation. A certain number of failed deconvolutions and misannotations will unfortunately occur, in particular of low abundant lipids, and hence manual curation is still required.
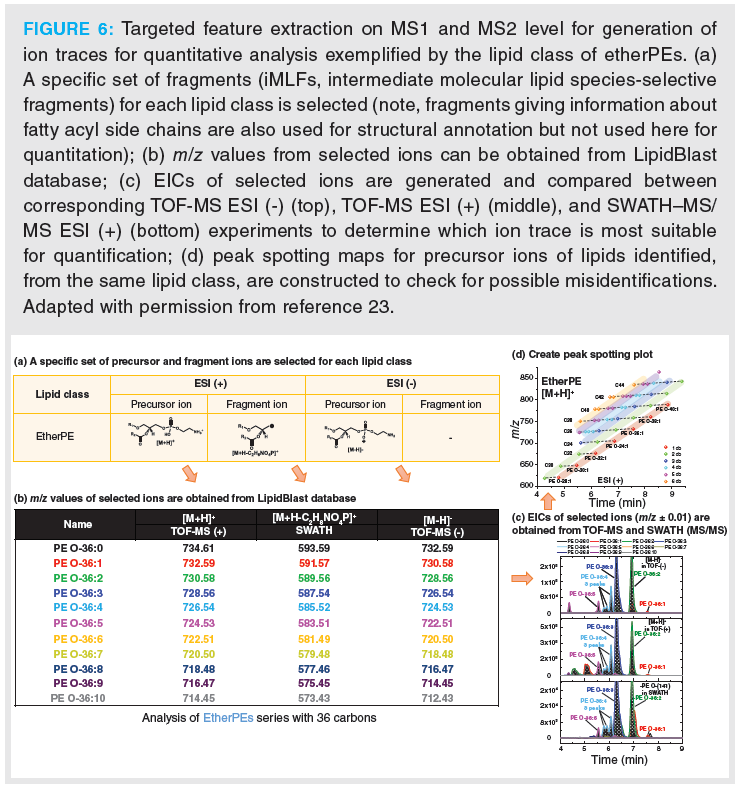
The second approach is targeted feature extraction (Figure 6). Lipids are well known, can be found in databases (for example, LipidMaps) or constructed from building blocks in a combinatorial chemistry approach (head groups plus fatty acyl side chains) using bioinformatics. It allows, by simple calculations, the creation of a peak list which can be used to extract features from the untargeted data file from vendor software. In the case of SWATH data, this can be done on MS1 and MS2 level. A specific set of precursor and fragment ions (preferentially intermediate molecular lipid species‑selective fragments [iMLF]) for each lipid class are selected (Figure 6[a]) and m/z values can be calculated or extracted from the LipidBlast database. With the list of m/z values (Figure 6[b]) EICs can be extracted from TOF‑MS and SWATH–MS/MS experiments in positive and negative ion mode (Figure 6[c]). The signals without interferences are then selected for quantitative analysis. For quantitative analysis with MS/MS data we choose fragments which are iMLFs since it is also possible to use them for peak spotting plots and get regular patterns according to their chromatographic elution. This cannot be done with smaller fragments containing only one FA chain. However, for the identification also fragments giving information about fatty acyl side chains are used for confirming structural annotation (see Supplemetary Information of reference 23). Furthermore, correct structural annotation is finally confirmed by creating peak spotting plots as shown in Figure 6[d]. They allow the location of misannotations quickly by irregular patterns of the retention time in the homologous lipid series.
In general, the workflow with automated lipid identification by database search using MS–DIAL provides higher identification numbers, however, also a larger number of misannotations. Manual curation is certainly required at present. By contrast, the data processing approach with targeted feature extraction based on sets of specific precursor and fragment ions gives more robust data but less identified lipids and is more time consuming.
Applications
A specific SWATH design covering the m/z range up to 1000 in MS full scan and m/z 30–500 with SWATH–MS/MS (19 SWATH windows with m/z width of 25 u) was employed for free fatty acid profiling in marine (Wakame) algae extract (25). Data processing with MS–DIAL resulted in 23 positively annotated fatty acids in negative ESI mode. Since fatty acids do not fragment, their identification was mainly based on MS1 similarity (that is, mass accuracy), isotope similarity and retention pattern on the peak spotting plot. Fatty acids found in the Wakame extract comprised typical marine polyunsaturated fatty acids (PUFAs) like ω-3 fatty acids docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), FA(18:3) n-3 and FA(16:4) n-3. However, FAs with uneven chain, such as FA(17:0) and FA(19:0), were also detected. For fatty acids with a carbon chain length of 16 and 18 the full series of degree of unsaturation from 0 to 4 was observed and for C20 the full series from 0 to 6 double bonds.
The lipidome of keratinocytes was characterized and 611 lipid species from 21 different lipid classes could be identified (23). It was most remarkable to find cholesterylesters (CEs) with fatty acyl chains from 16 to 36 carbons and up to 7 double bonds; usually CEs have acyl chains no longer than C20. They may represent a pool of building blocks for biosynthesis of long-chain ceramides which have been described as characteristic epidermal lipids playing an important role in the skin barrier. In a pharmacolipidomics approach, the effect of treatment of keratinocytes with betulin, a pentacyclic triterpene with mid-dermal wound healing properties, showed significant changes in 440 lipid species, amongst others a downregulation of cholesteryl esters and triacylglycerides and up-regulation of glycerophospholipids, sphingolipids, and diacylglycerides. The alteration in CEs could reflect the transesterification or consumption of ultra‑long chain FA pool for sake of remodelling of lipids involved in skin barrier.
Comprehensive lipid profiling by core–shell C8-RP UHPLC-ESI-QTOF-MS/MS using SWATH DIA was performed for the study of platelet lipidomes in coronary artery disease (CAD) (ST‑elevated myocardial infarction, stable angina pectoris, and healthy controls) (18). In this work, a variable SWATH window design with narrower isolation width in m/z ranges in which many lipids can be found, was combined with a targeted approach through programming narrow 5 u Q1 precursor isolation windows for a few oxidized phospholipids which were of prime interest. It provided the guarantee of assay specificity and lower LODs by increasing accumulation times for these targets. In total 611 lipids were identified (31% of all features) based on precursor mass accuracy, the match of isotope pattern and MS/MS spectra; only 34 features (2%) showed an MS precursor match but without MS/MS data for which reason these 2% were considered as not identified. Significant differences in the platelet lipidome of CAD patients compared to matched healthy donors were observed. For instance, lysophosphatidylinositol LPI(18:1) was significantly increased in the disease groups compared to the control group.
A combined targeted/untargeted SWATH design was also employed in a platelet lipidomics study of the supernatants of resting (non‑activated) and thrombin-activated platelets (24). Primary platelet lipid mediators such as thromboxane B2 (TXB2), 12S-hydroxy-5Z,8E,10E-heptadecatrienoic acid (HHT) and its oxidation product 12-keto-5Z,8E,10E-heptadecatrienoic acid (KHT) were programmed with narrow SWATH windows and untargeted profiling was also carried out by additional SWATH windows covering in particular the m/z range of (oxidized) fatty acids. The targeted analytes KHT, HHT, and TXB2 were found at highly elevated levels in the activated platelet releasates, including many PUFAs which were covered by the untargeted profiling. The results provided a more comprehensive picture of thromboinflammation than focusing interpretation solely on the primary platelet activation biomarker thromboxane.
A particular challenge in lipidomics is accurate quantitation of a large number of lipids based on a limited number of calibrants. Usually, a single stable isotope‑labelled internal standard or odd-chain internal standard for each lipid class is used for one-point calibration. The approach assumes that i) the calibration function is intersecting the origin, ii) the analyte concentration is within the linear range, iii) no weighted regression is required, and iv) the response factor is the same for all lipids of the class regardless of fatty acyl side chain and double bond number. This is usually not the case and hence the results of such a one‑point calibration are of limited accuracy. SWATH provides quantifiable data on the MS and MS/MS level, which can lead to increased assay specificity and better quantitative performance. Its potential for large scale lipidome quantification using a surrogate calibration with lipid class‑specific stable isotope labelled standards (due to lack of blank matrix) was demonstrated in the context of a mouse plasma lipidomics study (22). LLOQs in the range of 10–50 ng/mL for lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylglycerol (PG), sphingomyelin (SM), phosphatidylcholine (PC), phosphatidylethanolamine (PE), diacylglycerol (DAG), 100–700 ng/mL for monoacylglycerol (MAG), triacylglycerol (TAG), and 1–20 µg/mL for cholesterol and cholesterylester (CE) were obtained. To increase the accuracy for target lipids, response factors to corresponding surrogate calibrants were determined using a standard reference material (NIST SRM 1950), for which interlaboratory consensus values of numerous lipid species are published (32). In brief, by spiking and serial dilution of the certified reference material (CRM), linear calibration slopes of target lipid species and surrogate calibrants were constructed. Matrix-matched response factors could then be derived by division of slopes (target lipid species/corresponding surrogate calibrant). They were then applied for post-acquisition re-calibration, involving response factors, to yield improved accuracy in comparison to single-point calibration. While for low abundant lipids sensitivity is an issue, for highly abundant lipids the linear range is a critical parameter. Upper limits of quantitation (ULOQs) are typically in the range of 2–200 µg/L depending on the lipid class and ion type used for quantitation (22). Through the use of a less abundant fragment ion, in particular cases the linear range may be extended by use of two distinct ions for quantitation. The method was employed to obtain quantitative lipid results of plasma samples from mice fed with high‑fat diet versus control diet; significantly altered lipid levels were found, for example, for some PIs and PEs.
The LC–MS method with SWATH acquisition was also utilized for lipidomic profiling of non-mineralized dental plaque and in vitro biofilms (33). Dental plaque is a structurally organized biofilm and its (lipid) composition may be altered with the settlement of pathogenic microorganisms. Low identification rates of detected features, indicating possibly presence of uncommon lipids in the samples which are not present in the mass spectral databases utilized for identification, and the presence of specific uncommon lipids with odd‑chain fatty acid side chains are some of the findings of this study.
Conclusion
Overall, the potential and peculiarities of SWATH–MS hyphenated with UHPLC for the profiling of lipidomes of various biological samples is documented in the literature by ample examples. SWATH is an advanced acquisition mode, particularly for lipidomics, where numerous isomeric structures which cannot be distinguished on the MS1 level, regardless of mass resolving power of the instrument, can be identified.
References
- T. Züllig, M. Trötzmüller, and H.C. Köfeler, Anal. Bioanal. Chem.412(10), 2191–2209 (2020).
- I. Willenberg, A.I. Ostermann, and N.H. Schebb, Anal. Bioanal. Chem.407(10), 2675–2683 (2015).
- J. Wang and X. Han, TrAC Trends Anal. Chem.121, 115697 (2019).
- D. Wolrab, M. Chocholoušková, R. Jirásko, O. Peterka, and M. Holčapek, Anal.Bioanal. Chem. 412(10), 2375–2388 (2020).
- T. Cajka and O. Fiehn, Metabolomics 12(2), 34 (2016).
- H. Tsugawa, T. Cajka, T. Kind, Y. Ma, B. Higgins, K. Ikeda, M. Kanazawa, J. VanderGheynst, O. Fiehn, and M. Arita, Nat. Methods 12(6), 523–526 (2015).
- K. Eder, J. Chromatogr. B. 671(1), 113–131 (1995).
- M. Raetz, R. Bonner, G. Hopfgartner, Metabolomics 16 (6), 71 (2020).
- T.P.I. Lintonen, P.R.S. Baker, M. Suoniemi, B.K. Ubhi, K.M. Koistinen, E. Duchoslav, J.L. Campbell, and K. Ekroos, Anal. Chem. 86(19), 9662–9669 (2014).
- G. Paglia, P. Angel, J.P. Williams, K. Richardson, H.J. Olivos, J.W. Thompson, L. Menikarachchi, S. Lai, C. Walsh, A. Moseley, R.S. Plumb, D.F. Grant, B.O. Palsson, J. Langridge, S. Geromanos, and G. Astarita, Anal. Chem. 87(2), 1137–1144 (2015).
- I. Blaženović, T. Shen, S.S. Mehta, T. Kind, J. Ji, M. Piparo, F. Cacciola, L. Mondello, and O. Fiehn, O., Anal. Chem. 90(18), 10758–10764 (2018).
- C.G. Vasilopoulou, K. Sulek, A.-D. Brunner, N.S. Meitei, U. Schweiger‑Hufnagel, S.W. Meyer, A. Barsch, M. Mann, and F. Meier, Nat. Commun. 11(1), 331 (2020).
- Q. Xuan, C. Hu, D. Yu, L. Wang, Y. Zhou, X. Zhao, Q. Li, X. Hou, and G. Xu, Anal. Chem. 90(12), 7608–7616 (2018).
- J. Zhou, C. Liu, D. Si, B. Jia, L. Zhong, and Y. Yin, . Anal. Chim. Acta 972, 62–72 (2017).
- L.C. Gillet, P. Navarro, S. Tate, H. Röst, N. Selevsek, L. Reiter, R. Bonner, and R. Aebersold, Mol. Cell. Proteomics 11(6), O111.016717 (2012).
- Y. Zhang, A. Bilbao, T. Bruderer, J. Luban, C. Strambio‑De‑Castillia, F. Lisacek, G. Hopfgartner, E. Varesio, J. Proteome Res. 14(10), 4359–4371 (2015).
- B. Drotleff, M. Hallschmid, M. Lämmerhofer, Anal. Chim. Acta 1022, 70–80 (2018).
- J. Schlotterbeck, M. Chatterjee, M. Gawaz, M. Lämmerhofer, Anal. Chim. Acta 1046, 1–15 (2019).
- M. Raetz, E. Duchoslav, R. Bonner, and G. Hopfgartner, Anal. Bioanal. Chem. 411(22), 5681–5690 (2019).
- M.A. Moseley, C.J. Hughes, P.R. Juvvadi, E.J. Soderblom, S. Lennon, S.R. Perkins, J.W. Thompson, W.J. Steinbach, S.J. Geromanos, J. Wildgoose, J.I. Langridge, K. Richardson, and J.P.C. Vissers, J. Proteome Res. 17(2), 770–779 (2018).
- C. Messner, V. Demichev, N. Bloomfield, G. Ivosev, F. Wasim, A. Zelezniak, K. Lilley, S. Tate, and M. Ralser, bioRxiv 656793 (2019).
- B. Drotleff, J. Illison, J. Schlotterbeck, R. Lukowski, and M. Lämmerhofer, Anal. Chim. Acta 1086, 90–102 (2019).
- C. Calderón, L. Rubarth, M. Cebo, I. Merfort, and M. Lämmerhofer, Proteomics 20(11), 1900113 (2020).
- M. Cebo, J. Schlotterbeck, M. Gawaz, M. Chatterjee, and M. Lämmerhofer, Anal. Chim. Acta 1094, 57–69 (2020).
- J. Schlotterbeck, A. Kolb, and M. Lämmerhofer, J. Sep. Sci. 41(23), 4286–4295 (2018).
- J. Schlotterbeck, M. Cebo, A. Kolb, and M. Lämmerhofer, Anal. Bioanal. Chem. 411(2), 479–491 (2019).
- A. Gil, W. Zhang, J.C. Wolters, H. Permentier, T. Boer, P. Horvatovich, M.R. Heiner-Fokkema, D.-J. Reijngoud, and R. Bischoff, Anal. Bioanal. Chem. 410(23), 5859–5870 (2018).
- V. Matyash, G. Liebisch, T.V. Kurzchalia, A. Shevchenko, and D. Schwudke, J. Lipid Res. 49(5), 1137–1146 (2008).
- C. Calderón, C. Sanwald, J. Schlotterbeck, B. Drotleff, and M. Lämmerhofer, Anal. Chim. Acta 1048, 66–74 (2019).
- M.H. Sarafian, M. Gaudin, M.R. Lewis, F.-P. Martin, E. Holmes, J.K. Nicholson, and M.-E. Dumas, Anal. Chem. 86(12), 5766–5774 (2014).
- T. Kind, K.-H.Liu, D.Y. Lee, B. DeFelice, J.K. Meissen, and O. Fiehn, Nat. Methods 10(8), 755–758 (2013).
- J.A. Bowden, A. Heckert, C.Z. Ulmer, et al., J. Lipid Res. 58(12), 2275–2288 (2017).
- B. Drotleff, S.R. Roth, K. Henkel, C. Calderón, J. Schlotterbeck, M.A. Neukamm, and M. Lämmerhofer, Anal. Bioanal. Chem. 412(10), 2303–2314 (2020).
AUTHORS
Bernhard Drotleff is Assistant Professor at the Institute of Pharmaceutical Sciences at the University of Tübingen, Germany. During his PhD in the Lämmerhofer group he developed LC–MS methods for steroid analysis, large scale lipid quantification, and bioinformatics tools for normalization in lipoidomics. His research interests are bioanalytical mass spectrometry, analytical bioinformatics, steroidomics and lipidomics.
Carlos Calderón Castro is currently an Invited Professor at the Chemistry School of the University of Costa Rica, Costa Rica. He received his PhD in 2020 from the Institute of Pharmaceutical Sciences at the University of Tübingen, in Germany under the supervision of Prof. Dr. Michael Lämmerhofer. His PhD work was based on the development of novel methodologies for the analysis of lipids by HPLC-MS.
Malgorzata Cebo is currently working at the Shared Research Facility Metabolomics in Vienna BioCenter, Austria. She is also finalizing her PhD studies from the University of Tübingen, where she worked on developing analytical methods for studying lipids and oxylipins in human platelets.
Kristina Dittrich is a PhD student at the University of Tübingen under the supervision of Prof. Lämmerhofer. Her research field is the clinical lipidomics of human platelets performing untargeted lipidomics by the use of UHPLC–MS/MS methods.
Xiaoqing Fu is a PhD student at the University of Tübingen, in Prof. Lämmerhofer’s work group. Her PhD work is based on developing LC–MS methods for oxylipins and lipids, and performing untargeted lipidomics on human platelets and plasma. Her research interests are lipidomics and clinical bioanalysis.
Michael Lämmerhofer is Professor for Pharmaceutical (Bio-)Analysis at the University of Tübingen, Germany. His research interests include chiral separations, multidimensional liquid chromatography, clinical bioanalysis and lipidomics, oligonucleotide and biopharmaceuticals analysis as well as functionalized separation materials.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.










