Trends in Enantioselective High Performance Liquid Chromatography
Special Issues
Enantioselective high performance liquid chromatography (HPLC) is slowly adopting the modern particle technologies (sub-2-µm fully porous particles [FPPs] and sub-3-µm superficially porous silica particles [SPPs]) that have been well known in reversed-phase LC for the past decade. The most significant benefit is that enantiomer separations can be performed much faster, which is of interest in high-throughput screening applications and multidimensional enantioselective HPLC analysis. The state of the art is briefly discussed with some examples documenting the potential of core–shell particle technology and comprehensive multidimensional separations.
Ulrich Woiwode1, Stefan Neubauer1, Mike Kaupert1, Wolfgang Lindner2,3, and Michael Lämmerhofer1,1Institute of Pharmaceutical Sciences, Pharmaceutical (Bio-)Analysis, University of Tübingen, Tübingen, Germany, 2Lindner Consulting GmbH, Klosterneuburg, Austria, 3Institute of Analytical Chemistry, University of Vienna, Vienna, Austria
Enantioselective high performance liquid chromatography (HPLC) is slowly adopting the modern particle technologies (sub-2-µm fully porous particles [FPPs] and sub-3-µm superficially porous silica particles [SPPs]) that have been well known in reversed-phase LC for the past decade. The most significant benefit is that enantiomer separations can be performed much faster, which is of interest in high-throughput screening applications and multidimensional enantioselective HPLC analysis. The state of the art is briefly discussed with some examples documenting the potential of core–shell particle technology and comprehensive multidimensional separations.
Chiral separation by liquid chromatography (LC) is an enabling technology in drug discovery, clinical diagnosis, food and agrochemistry, environmental analysis, and many other fields (1,2). Researchers can now select from a variety of chiral stationary phases (CSPs) and commercial columns, which allow the separation of enantiomers of virtually any kind of chiral compound. However, most commercially available CSPs are still based on 5-µm particles. Efficiencies are lower and analysis times are usually longer than analyses with reversed-phase LC with modern columns. The introduction of CSPs based on 3-µm silica particles occurred when ultrahigh-pressure liquid chromatography (UHPLC) was already an established technology in reversed-phase LC. For example, polysaccharide CSPs based on 3-µm particles were launched in 2008 (3). Traditionally, in the field of chiral separation, the focus of research was on the search and development of new chiral selectors with broad enantioselectivity (Table 1, top). The engineering of the underlying support and the column technology lagged behind the developments in achiral LC and have only become the subject of intensive research in recent years.
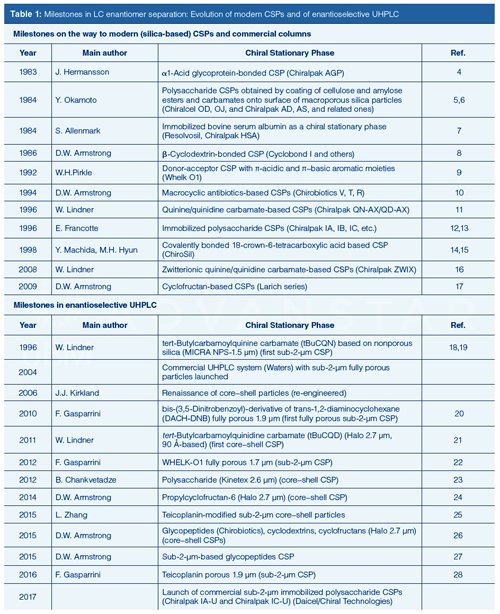
Historical Development of Enantioselective UHPLC
The history of LC in the last century was accompanied by a constant decrease in particle diameter of the supporting (silica) particles. In the 1990s, small-sized silica particles were already commercially available, but adequate HPLC equipment for their practical application at full potential was still missing. For example, in the late 1980s and early 1990s nonporous silica particles with diameters of 1.5 µm and 1 µm were available (29). Such materials with alkyl surface bondings were explored in particular for protein separations. The idea was to eliminate intraparticular pore diffusion, which is very slow for such macromolecular analytes. Although these reversed phase stationary phases were not very successful for small molecule LC separations, they have been tested as supports for fast and highly efficient enantiomer separations. After modification of these particles with quinine carbamate ligands (Figure 1[a]), they were evaluated for fast enantiomer separation of chiral acids (18,19,30). An example chromatogram is given in Figure 1(b). The enantiomers of 3,5-dinitrobenzoyl-leucine could be baseline resolved within 1.5 min. Through elimination of the mesopores in the silica particles, the surface area was about a factor 100 lower than on 100-Å fully porous silica particles commonly used for small molecule separations and brushâtype chiral selectors. This, in combination with the short columns used (33 × 4.6 mm), gave rise to fast enantiomer separations. The high efficiencies expected from the small particle diameter could unfortunately not be fully achieved because of deleterious extra-column effects. UHPLC equipment with reduced extra-column contributions to peak broadening was not available at that time. A number of other chiral compounds were also separated with short run times (< 2 min).
When commercial UHPLC equipment was introduced in 2004, sub-2-µm silica particles for highly efficient separations became the standard in reversed-phase LC. Somewhat later, core–shell particles, re-engineered by J.J. Kirkland et al., experienced a renaissance for small molecule separations and emerged as a competitor technology to subâ2âµm particles in UHPLC. However, it still took years until the first attempts were made to introduce them for chiral separations.
In 2010, Gasparrini and co-workers reported on a brush-type sub-2-µm chiral stationary phase obtained by covalent grafting of the bis-(3,5-dinitrobenzoyl)-derivative of trans-1,2-diaminocyclohexane (DACH-DNB) (20). Such Pirkleâtype CSPs are deemed to have fast adsorption–desorption kinetics during the enantiorecognition process and are thus regarded as beneficial selector systems for fast and highly efficient enantiomer separations on subâ2âµm particles. The performance of 1.9-µm fully porous particle (FPP) based DACH-DNB chiral stationary phases was compared to analogs with corresponding 4.3- and 2.6-µm particle size CSPs. Van Deemter plots for the three DACH-DNB CSPs packed into 100 × 4.1 mm columns using 90:10 (v/v) hexane–CHCl3 as eluent at a column temperature of 25 °C and methyl benzoate as a test solute revealed minimal plate heights Hmin of 10.5-, 6.7-, and 5.2âµm for the columns packed with 4.3-, 2.6-, and 1.9-µm DACH-DNB CSPs, corresponding to reduced plate heights h of 2.4, 2.6, and 2.8, respectively, at optimal flow velocities of 1.6-, 3.1-, and 4.0-mm/s (20). Although this is slightly higher h than for reversed-phase type materials and corresponding separations, respectively, it indicated the potential of the technology. Several separations of enantiomeric pairs with analysis times in the 15–40 s range could be achieved on the sub-2-µm FPP chiral stationary phase. Comparison of column performances on HPLC and UHPLC systems using benzyl phenyl sulfoxide as probe yielded 6500 theoretical plates for each column (50 × 4.1 mm) and documented that resolution is lost (37%) when common HPLC equipment with excessive extra-column volumes is used for such separations with highly efficient columns. At about the same time, Ai et al. reported on sub-1-µm size mesoporous silica particles derivatized with perphenylcarbamoylated-β-cyclodextrin (32 µmol/g) (31). While relatively fast separations were achieved (< 10 min) on a 50 × 4.6 mm column, plate numbers in the order of 70,000/m resembled those of standard enantioselective HPLC separations.
The first report on a core–shell (also termed superficially porous or fused-core) particle-based CSP was published by Lindner and co-workers in 2011 (21). Superficially porous particles (SPP) (particle diameter 2.7 µm, 1.7 µm solid core with a 0.5âµm porous layer 90 Å) were modified with tert-butylcarbamoylquinidine and packed into 50 × 4 mm columns. A set of 19 racemic amino acids derivatized with 3-ferrocenylpropionyl-hydroxysuccinimide were successfully separated by LC coupled to tandem mass spectrometry (MS/MS) within 8 min (21). Although this work did not evaluate the efficiency of this new particle type in comparison to 5-µm fully porous standard materials, it was evident that better efficiencies and faster separations can be achieved with this chiral adsorbent.
Polysaccharide CSPs have become the most widely used adsorbents for enantiomer separations by HPLC in industry. These macromolecular chiral selectors are coated onto wide-pore (100-nm pore size) silica particles. The wide pores assure the homogeneous coating of a thin film of chiral selector and avoid clogging of the pores. Superficially porous silica particles with such wide pores were not available until recently. Thus, the first attempts were made with core–shell silica of 2.6-µm nominal particle diameter and 9-nm nominal pore size (23). Cellulose tris(4-chloro-3-methylphenylcarbamate) was coated onto the surface of these particles and compared to corresponding CSPs based on 3-µm wide pore silica particles (commercial column) as well as 3-µm totally porous silica with comparable pore size (10 nm), all packed into 250 × 4.6 mm columns. On the two narrow pore columns, only 5% (w/w) of polysaccharide selector could be coated without problems and this reduced separation factors significantly when compared to the commercial 3-µm column with about 4 times more chiral selector. Higher separation factors (probably a result of more selector per unit m2; the totally available surface area is lower for SPP versus FPP because of the solid core) but flatter H/u curves were reported for the core–shell particle CSPs. Later, SPPs with 3.6 µm nominal particle diameter and 20-, 30-, and 60-nm nominal pore size, respectively, were coated with cellulose tris(3,5-dimethylphenylcarbamate). Only 2% (w/w) selector (related to silica particles) were coated since with 5% (w/w) selector pores appeared to be already partly clogged (32). Reduced plate heights in the order of 1.4 to 1.7 could be achieved at optimal (low) flow rates for the first eluted enantiomers indicating that SPP CSPs with macromolecular selectors such as polysaccharides can also yield highly efficient enantiomer separations; however, this is potentially at the expense of separation factors if compared to commercial standard materials (wide pore with ca. 20% selector).
Armstrong and co-workers have published a series of papers in which they describe the transfer and evaluation of their chiral selector technologies (glycopeptide macrocyclic antibiotics, cyclodextrins, and cyclofructan derivatives) on SPPs (26). The focus was mostly on the speed of enantiomer separations and will be discussed below.
The first columns packed with subâ2-µm FPP-based polysaccharide CSPs were launched this year. It will be interesting to see how this new particle technology will be appreciated by the users in industry. However, it may stimulate and accelerate the transition from enantioselective HPLC to enantioselective UHPLC.
Evaluation of Superficially Porous Chiral Anion-Exchanger CSPs
Detailed characterizations of the performance of various SPP and FPP CSPs were performed by Gasparrini et al. (33–35). In the following discussion, the focus is on chiral anion-exchanger CSPs based on core–shell particles (SPPs).
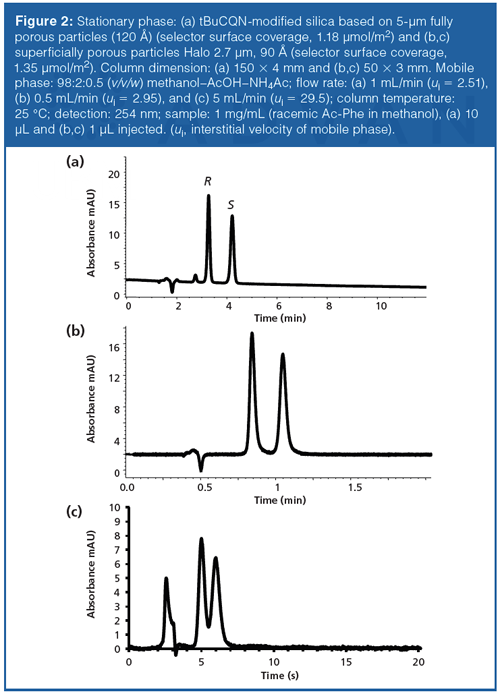
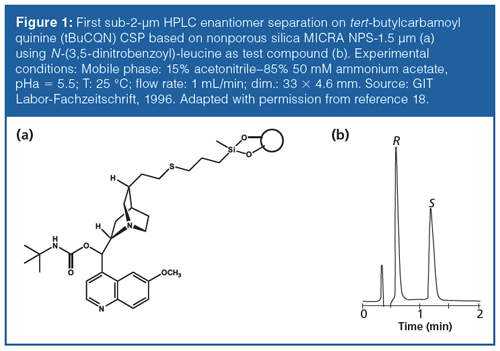
Figure 2 shows the comparison of the enantiomer separation of N-acetyl-phenylalanine (Ac-Phe) on tert-butylcarbamoylquinine CSP based on (a) fully porous 5-µm silica particles (120 Å) (150 × 4 mm) and (b) core–shell 2.7-µm particles with a 0.5-µm porous shell (90 Å) (50 × 3 mm). It can be seen that the separation can be readily transferred to such core–shell particles, as reported previously (21,36). Essentially the same separation factors can be achieved on the core–shell particles (α = 1.51 versus 1.49 on 5-µm 120 Å fully porous versus 2.7-µm superficially porous 90 Å CSPs). The faster separations on the core–shell particle column in Figure 2(b) are a combined effect of the reduced total surface area (ca. factor 2.7 lower specific surface area) as well as a shorter column length (50 versus 150 mm in the case of the fully porous particle column). It should be noted that the core–shell particles have been packed into a narrower 3-mm internal diameter (i.d.) column (versus 4-mm i.d. for the fully porous particle CSP), which is more favourable for electrospray ionization (ESI)-MS hyphenation in terms of sensitivity because it can be used with lower volumetric flow rate at identical linear velocities. Such smaller dimension columns are more difficult to pack with good quality unfortunately, yet 3 mm is a good compromise.
The number of theoretical plates per m could be increased by a factor of 2 or so, when switching from the 5 µm FPP (120 Å; 150 × 4 mm) (Figure 2[a]) to 2.7 µm SPP (90 Å; 50 × 3 mm) support (Figure 2[b]) (at comparable interstitial linear velocities; ui = 2.51 versus 2.95 mm/s). This was less than reported previously by D.W. Armstrong (factor 3) (36). It is also quite obvious from Figure 2(b) and 2(c) (where the same SPP support is operated at a higher interstitial flow velocity of 29.5 mm/s) that there is a significant loss in plate numbers at higher flow velocities. Indeed, if we look at the dependency of the efficiency (plate height H) on the interstitial linear flow velocities (H/ui-curves) (Figure 3), we notice that the minimum is only reached at very low flow velocities and the slope of the C-term branch is not negligible, even at elevated temperatures. While the slope is flatter for the 2.7-µm SPP than for the 5-µm FPP with a bonded chiral selector (36), contributions from peak broadening because of mass transfer resistance is more significant in enantioselective UHPLC (independent of the particle type) as a result of slow adsorption–desorption kinetics when compared to reversed-phase LC (37). The underlying kinetics of affinityâtype multiple attachment of analyte at the chiral selector binding site is several orders of magnitude slower compared to reversed phase-type adsorption–desorption processes and thus affects the overall mass transfer resistance. It was estimated to contribute up to about 30% to the total HETP (37). Minimal reduced plate heights hmin approximate 2 at optimal velocities ui,opt < 1 mm/s (Figure 3 and reference 36). Whilst further improvements in terms of column packing and stationary phase design should be possible, the core–shell CSP technology promises significant potential in chiral separations, as already documented previously by the above cited works.
Superficially Porous versus Fully Porous Particle-Based CSPs
In the literature it is well documented for reversed-phase columns that superficially porous sub-3-µm particle packed columns perform equally well as fully porous sub-2-µm ones at the benefit of lower back pressures. Thus, manufacturers of chiral CSPs and enantioselective columns may ask which one of the supports-FPP sub-2-µm or SPP subâ3-µm-is the preferred choice for new column developments.
Gritti and Guiochon presented a theoretical approach to answer this question (37). Considering slow rates of adsorption–desorption onto the CSP in enantioselective HPLC (kads 1000 s-1 versus 107-8 s-1 in reversedâphase LC), and assuming a single site adsorption mechanism, a reduced HETP equation with 4 terms based on a Laplace transform of the general rate model of chromatography was used to model h/ν-curves for superficially porous particles as support (dcore/dparticle = 0.7) and for comparison with fully porous particles as carrier using trans stilbene oxide as model analyte. Physicochemical parameters were either accurately measured or best approximated. The calculations were based on a complete set of actual kinetic parameters (longitudinal diffusion, eddy dispersion, intraparticle diffusivity, and adsorption–desorption constant) measured for a reference column packed with a cellulose carbamate CSP based on FPPs. The results of the calculations revealed that columns packed with core–shell particles may outperform those packed with FPPs under the assumption that both are well packed. The reduced plate height could be decreased to a minimum from 2.0 with fully porous to 1.7 with core–shell particles. A maximal gain in resolution of ca. 10% was postulated, which is not much but also not negligible if the separation is difficult.
Armstrong and Gasparrini addressed this question experimentally. The results were controversial. The bottom line was that 2.1-µm FPP-based cyclofructan CSP showed higher resolution than a corresponding 2.7-µm SPP-based CSP under identical conditions, however, under isoeluotropic conditions, the core–shell CSP outperformed the FPP-based CSP (38). The efficiency was higher on the superficially porous particles in both instances. Gasparrini, Cavazzini, and co-workers compared columns packed with Pirkle-type 1-(3,5-dinitrobenzamido)-1,2,3,4-tetrahydrophenanthrene-based CSPs based on different supports, viz. 1.8 µm and 2.5 µm FPPs as well as 2.6 µm SPPs (39). Contrary to the results from Armstrong, the columns packed with 2.6-µm SPPs and 2.5âµm FPPs CSPs exhibited similar efficiencies (first eluted enantiomer) or even slightly worse efficiency for the second eluted enantiomer on the SPP column. More significant eddy dispersion or slow adsorption–desorption kinetics or both were made responsible for the worse behaviour of SPPs in the case of the second eluted enantiomer (note, a more dense selector coverage was observed for the SPP CSP with narrower pores compared to FPP CSPs).
It remains unclear what is the reason for the contradictory conclusions of the two groups. However, it is evident that they used distinct chiral selectors, which may behave quite differently in terms of adsorption–desorption rate constant. Pirkle-type selectors, such as 1-(3,5-dinitrobenzamido)-1,2,3,4-tetrahydrophenanthrene-based CSP, are known to have relatively fast adsorption–desorption kinetics, while cyclofructans involve multiple hydrogen bonding-driven inclusion complexation, which might be associated with substantially lower adsorption–desorption rate constants. To what extent system effects and sub-optimal column packings contribute to the contrary results remains unclear as well. Some uncertainties may also arise from distinct selector surface coverages in SPP- and FPP-based columns as well as SPPs from distinct suppliers with different pore diameters (for example, 80 versus 120 Å) and different pore diffusivity, which may affect kinetic behaviour differently.
Fast Enantiomer Separations
The quest for fast enantiomer separations was previously driven by high-throughput applications such as chiral drug screening and chiral catalyst development (35). Domains for fast chiral separations have been microfluidic chip-based enantioselective separations (40) and SFC enantiomer separations (41). With the establishment of sub-2-µm fully porous CSPs as well as superficially porous CSPs, enantioselective LC is capable of separations in seconds and was recently promoted by the groups of Gasparrini (20,28,42) and Armstrong (38,43). Some instrumental requirements, such as a request for high detector sampling rates, an absence of excessive extra-column effects, and an absence of radial temperature gradients from frictional heating, have to be fulfilled so that the resolution in such extremely fast separations is not lost (38).
Subminute enantiomer separations can nowadays be routinely achieved on sub-2-µm nonporous (see Figure 1[b]) (18,30) and FPP CSPs (20,42) as well as on sub-3-µm SPP based CSPs (Figure 2[b]) (38) packed into 3–5 cm long columns. Besides short columns, high flow rates are required. For example, Figure 2(b) and 2(c) show enantiomer separations of Ac-Phe on a 5-cm long (3 mm i.d.) column packed with tBuCQN-modified superficially porous 2.7-µm CSP at a flow rate of 0.5 mL/min (Figure 2[b]) and 5 mL/min (Figure 2[c]). This accelerates the separation from a minute timescale to a 6 s timescale. Since linear (interstitial) flow velocities, ui, of around 29.5 mm/s resulted, mass transfer limitations have led to some loss in resolution (Rs declined from 3 at 0.5 mL/min to 1.1 at 5 mL/min). At such high speed, extraâcolumn effects may become more critical. The UHPLC instrument has not been particularly optimized to reduce extraâcolumn band broadening, yet a higher detector sampling rate of 160 Hz has been used.
To further speed up the separations, temperature can be elevated (Figure 4). In enantiomer separations, this typically has a detrimental effect on enantioselectivity since most separations are enthalpically dominated. However, the loss in enantioselectivity is compensated by the increase in efficiency, affording essentially the same resolution at all tested temperatures (Figure 4).
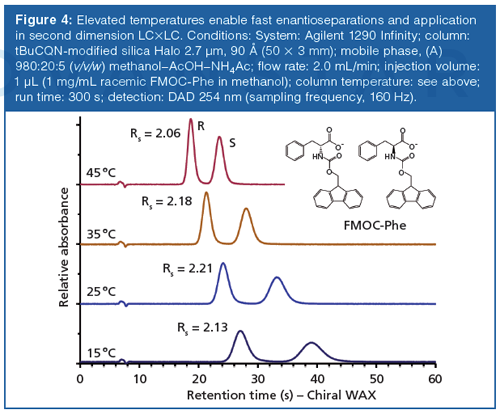
More recently, the limits have been pushed further and ultrafast separations in the timescale of less than a second, achieved by even shorter columns and higher flow rates, have been reported by Armstrong (0.5 × 4.6 mm, 5 mL/min flow rate) (44) and Gasparrini (10 × 3 mm, 8 mL/min flow rate) (33). Such subâsecond enantiomer separations open up the avenue for on-line 2D-HPLC enantiomer separations with the enantioselective column as the second dimension.
Comprehensive TwoâDimensional Enantioselective HPLC
The analysis of complex mixtures is gaining more and more impetus in analytical chemistry, and in fields that involve chiral compounds. A single dimension separation with CSPs is often not sufficient in many instances. It is well known that “chiral columns” have a low peak capacity, but even though they have a good enantioselectivity, they have limited chemoselectivity for the separation of structurally closely related substances. Achiral–chiral and chiral–achiral column coupling strategies have therefore been a commonly established approach in bioanalysis of chiral pharmaceuticals, such as the stereoselective analysis of parent drug and metabolites (45). Heart-cutting (46) and (multi-loop) multiple heart-cutting 2D-HPLC methods (47–49) have been utilized for this purpose. Such heartâcutting 2D-LC was also realized on miniaturized formats, for example, microfluidic chip with integrated fluorescence and MS detection (50).
More challenging is the implementation of a comprehensive enantioselective 2D-HPLC setup. Welsch and co-workers reported a feasibility study of comprehensive two-dimensional on-line HPLC enantiomer separation (30). In this study a narrow-bore C18 RP column (150 × 1 mm) in the first dimension was coupled to a short (33 × 4.6 mm) enantioselective column based on nonporous 1.5-µm silica particles derivatized with quinidine carbamate as chiral selector in the second dimension. A mixture of amino acids derivatized with 3,5-dinitrobenzoyl chloride was separated. Fraction transfer in comprehensive 2D-LC can be based on either alternately filling two loops (that is, sampling the first dimension eluate into a loop while the content of the second loop is analyzed in the second dimension) or by a stop–flow transfer technique (that is, the flow is stopped while the fraction from the first dimension is analyzed). In either case, a fast second dimension separation is required and was achieved in this case because of the nonporous support (enantiomer separations in less than 1.5 min). The stop–flow transfer technique was used and the overall analysis time for this two-dimensional separation of nine racemic DNB-amino acids was about 16 min.
With the advent of ultrafast enantiomer separations on core–shell and sub-2-µm particle CSPs, comprehensive 2D-HPLC along with the availability of commercial instrumentation for this purpose got another boost. The stop–flow transfer technique discussed above makes this technique slow, that is, the stop–flow technique increases the total analysis time. On the other hand, with the alternately two loop filling technique commonly adopted nowadays in comprehensive 2D-HPLC, the second dimension separation must be performed fast enough to avoid undersampling in the first dimension. According to the Murphy-Schure-Foley rule, (at least) four fractions (samples) per 8σ peak width should be collected (51), otherwise resolution already achieved in the first dimension separation will be lost. Hence, ultrafast separations in the second dimension are of utmost importance in 2D-LC separations. A fully comprehensive chiral × chiral 2D-LC–UV analysis of a mixture of isomers of a synthetic intermediate of hepatitis C virus protease inhibitor (anti-HCV therapeutic) was reported recently by Barhate et al. (49). In this specific case, two chiral columns were coupled, namely a narrow-bore column (150 mm × 2.1 mm packed with cellulose tris[4-methylbenzoate] 3 µm) in the first dimension combined with an ultrafast chiral separation on a 50 × 4.6 mm chiral column (packed with cellulose tris[3,5-dimethylphenylcarbamate], 3 µm) in the second dimension. The first dimension was run using a very slow gradient (130 min; flow rate: 50 µL/min), while the second dimension was run at a high flow rate of 3 mL/min. This allowed 40-µL fractions from the first dimension to be collected into a sample loop, corresponding to about 1 fraction sampled every 48 s. Its analysis was completed while the next fraction was sampled into a second loop. The UV detector was operated at a sampling frequency of 240 Hz to accurately measure the peaks with true peak shape.
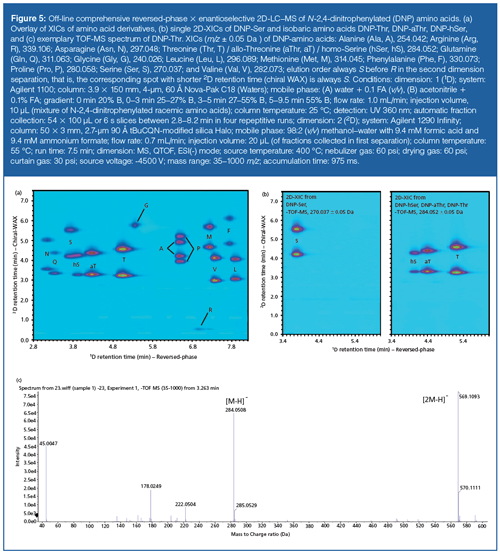
For many pharmaceutical, biopharmaceutical, and bioanalysis applications of 2D-LC, hyphenation to mass spectrometric (MS) detection is desirable. This poses some additional constraints. For example, the high volumetric flow rate (3 mL/min) required above to achieve fast second dimension separations is detrimental for ESI. Atmospheric pressure chemical ionization (APCI), which can tolerate higher flow rates, is less sensitive for most polar analytes. To cope with this problem, a flow splitting strategy could be adopted or the inner diameter of the second dimension column could be reduced (however, this requires readjustment of the first dimension column diameter as well). A simple and straightforward solution is to perform separations off-line. Figure 5 shows the comprehensive reversedâphase × enantioselective weak anion-exchange 2D-HPLC–ESIâQTOF-MS separation of an amino acid mixture derivatized with Sanger’s reagent. The 2,4-dinitrophenylated (DNP) amino acid mixture was first separated in the first dimension by reversed-phase LC allowing critical peak pairs such as isobaric amino acids (threonine, Thr; allo-threonine, aThr; and homoserine, hSer) to be resolved. Fifty-four fractions of 100 µL each or 6 s slices between 2.8–8.2 min were collected in the first dimension using automated fraction collection. They were then injected into a 50 × 3 mm tert-butylcarbamoylquinine (tBuCQN)-modified SPP packed column (based on 2.7-µm SPP, 90 Å) operated with a volatile mobile phase (52) at a flow rate of 0.7 mL/min, which is compatible with the ESI source of the QTOF instrument used. The MS detector was run in negative ESI(-) MS-TOF mode. Figure 5(a) shows the overlay of the extracted ion chromatograms of the different DNP-amino acids of the sample. It can be seen that the amino acid enantiomers are well resolved and the overall resolution originates from the combination of the two orthogonal selectivity principles. It is evident that enantiomers would not be resolved by reversed-phase LC alone, while in 1D enantioselective weak chiral anion-exchange chromatography, many overlaps of amino acid derivatives would occur as a result of insufficient chemoselecitvity. It is worthwhile highlighting that the three isobaric DNP-amino acid derivatives Thr, aThr, and hSer are fully resolved in reversedâphase LC × enantioselective weak anion exchanger because of their complete isobaric resolution in 1D (reversedâphase LC). Full resolution of the Asn (N)/Gln (Q) enantiomer pairs in 2D-LC is only possible as a result of the combined selectivity in both dimensions. On the other hand, the full resolution of the enantiomer pairs of Ala (A) and Pro (P) DNP derivatives is solely possible because of the 2D separation and its sufficient enantioselectivity as well as simultaneous chemoselectivity. The 1D separation, however, avoids overlap of these two enantiomer pairs with other components of the test mixture. The benefit of the 2D-LC setup is clearly evident. Moreover, its hyphenation with MS detection brings about another selectivity dimension through specific detection. For all the peaks (spots), high-resolution TOF-MS spectra are available, which allows the generation of compoundâspecific extracted ion chromatograms (XICs) (Figure 5[b]) and identification of the compounds by accurate mass (Figure 5[c]) (for example, precursor ion [M-H]- of DNP-Thr: m/z calculated = 284.0524; m/z found = 284.0508 for [M-H]-; mass accuracy = -5.9 ppm). The adaptation into a corresponding on-line 2D-LC method needs some further modifications, but should be readily possible.
Conclusions
Efficient UHPLC enantiomer separations can be achieved with CSPs based on superficially porous sub-3-µm silica particles. The favourable flat H/u-curves seen with reversed phase-type superficially porous particles vanishes as a result of slow adsorption–desorption kinetics. Minimal h-values of around 2 can be obtained, yet at low flow rates only. Packing of these adsorbents into narrow inner diameter columns is still challenging. To gain the full potential of such adsorbents, extraâcolumn effects have to be minimized. The discussion whether such subâ3âµm superficially porous particle columns can outperform subâ2-µm fully porous particle columns is controversial. Subâminute and subâsecond enantiomer separations have been achieved with superficially porous sub-3-µm particle columns. Such timescales are of interest for highâthroughput applications of enantiomer separation and enantioselective multidimensional separations. Enhanced fluidity chromatography (supercritical fluid chromatography [SFC]) with subâ2âµm and core–shell particles is also quickly developing in the field of enantioselective analysis, but was not covered herein, and with optimized equipment may have the potential to be even faster (36).
Short core–shell columns were evaluated in an off-line comprehensive reversed-phase LC × enantioselective 2D-HPLC–ESIâQTOF-MS setup for the analysis of a racemic amino acid mixture with isobaric constituents. The combination of the two selectivity principles complemented each other, that is, the achiral reversed phase separation dimension helped to overcome the limited chemoselectivity of the superficially porous chiral stationary phase providing overall enhanced selectivity. Such 2D-LC approaches could also be beneficial for peptide stereoisomer separations that could benefit from the complementary selectivity of chiral and reversed phase phases. The on-line hyphenation needs further acceleration of the second dimension chiral separation.
Acknowledgements
M.L. is grateful for support by the “Struktur- und Innovationsfonds Baden-Württemberg (SI-BW)” and the German Science Funds (DFG no. INST 37/821-1 FUGG).
References
- N.M. Maier, P. Franco, and W. Lindner, Journal of Chromatography A906, 3–33 (2001).
- M. Lämmerhofer, Journal of Chromatography A1217, 814–856 (2010).
- T. Zhang and P. Franco, LCGC Europe21 430–432, 434, 436–437 (2008).
- J.R. Hermansson, Journal of Chromatography A269, 71–80 (1983).
- Y. Okamoto, M. Kawashima, and K. Hatada, Journal of the American Chemical Society 106, 5357–5359 (1984).
- Y. Okamoto, M. Kawashima, K. Yamamoto, and K. Hatada, Chemistry Letters13, 739–742 (1984).
- S. Allenmark, B. Bomgren, and H. Borén, Journal of Chromatography A316, 617–624 (1984).
- D. Armstrong, T. Ward, R. Armstrong, and T. Beesley, Science232, 1132–1135 (1986).
- W.H. Pirkle, C.J. Welch, and B. Lamm, The Journal of Organic Chemistry57, 3854–3860 (1992).
- D.W. Armstrong, Y. Tang, S. Chen, Y. Zhou, C. Bagwill, and J.-R. Chen, Analytical Chemistry66, 1473–1484 (1994).
- M. Lämmerhofer and W. Lindner, Journal of Chromatography A741, 33–48 (1996).
- E. Francotte, Patent WO1996027615 A1, 12 September 1996.
- E. Francotte and T. Zhang, Journal of Chromatography A1467, 214–220 (2016).
- M.H. Hyun, J.S. Jin, and W. Lee, Journal of Chromatography A822, 155–161 (1998).
- Y. Machida, H. Nishi, K. Nakamura, H. Nakai, and T. Sato, Journal of Chromatography A805, 85–92 (1998).
- C.V. Hoffmann, R. Pell, M. Laemmerhofer, and W. Lindner, Anal. Chem.80, 8780–8789 (2008).
- P. Sun, C. Wang, Z.S. Breitbach, Y. Zhang, and D.W. Armstrong, Analytical Chemistry81, 10215–10226 (2009).
- M. Lämmerhofer and W. Lindner, GIT Special: Chromatogr. Int. 40, 16 (1996).
- V. Piette, M. Lämmerhofer, K. Bischoff, and W. Lindner, Chirality9, 157–161 (1997).
- G. Cancelliere, A. Ciogli, I. D’Acquarica, F. Gasparrini, J. Kocergin, D. Misiti, M. Pierini, H. Ritchie, P. Simone, and C. Villani, Journal of Chromatography A1217, 990–999 (2010).
- R.J. Reischl, L. Hartmanova, M. Carrozzo, M. Huszar, P. Frühauf, and W. Lindner, Journal of Chromatography A1218, 8379–8387 (2011).
- D. Kotoni, A. Ciogli, C. Molinaro, I. D’Acquarica, J. Kocergin, T. Szczerba, H. Ritchie, C. Villani, and F. Gasparrini, Analytical Chemistry84, 6805–6813 (2012).
- K. Lomsadze, G. Jibuti, T. Farkas, and B. Chankvetadze, Journal of Chromatography A1234, 50–55 (2012).
- D.A. Spudeit, M.D. Dolzan, Z.S. Breitbach, W.E. Barber, G.A. Micke, and D.W. Armstrong, Journal of Chromatography A1363, 89–95 (2014).
- Y. Min, Z. Sui, Z. Liang, L. Zhang, and Y. Zhang, Journal of Pharmaceutical and Biomedical Analysis 114, 247–253 (2015).
- D.A. Spudeit, Z.S. Breitbach, M.D. Dolzan, G.A. Micke, and D.W. Armstrong, Chirality27, 788–794 (2015).
- C.L. Barhate, M.F. Wahab, Z.S. Breitbach, D.S. Bell, and D.W. Armstrong, Analytica Chimica Acta898, 128–137 (2015).
- O.H. Ismail, A. Ciogli, C. Villani, M. De Martino, M. Pierini, A. Cavazzini, D.S. Bell, and F. Gasparrini, Journal of Chromatography A1427, 55–68 (2016).
- K.K. Unger, G. Jilge, J.N. Kinkel, and M.T.W. Hearn, Journal of Chromatography359, 61–72 (1986).
- T. Welsch, C. Schmidtkunz, B. Müller, F. Meier, M. Chlup, A. Köhne, M. Lämmerhofer, and W. Lindner, Analytical and Bioanalytical Chemistry388, 1717–1724 (2007).
- F. Ai, L. Li, S.-C. Ng, and T.T.Y. Tan, Journal of Chromatography A1217, 7502–7506 (2010).
- L. Bezhitashvili, A. Bardavelidze, T. Ordjonikidze, L. Chankvetadze, M. Chity, T. Farkas, and B. Chankvetadze, Journal of Chromatography A 1482, 32–38 (2017).
- M. Catani, O.H. Ismail, F. Gasparrini, M. Antonelli, L. Pasti, N. Marchetti, S. Felletti, and A. Cavazzini, Analyst142, 555–566 (2017).
- A. Cavazzini, N. Marchetti, R. Guzzinati, M. Pierini, A. Ciogli, D. Kotoni, I. D’Acquarica, C. Villani, and F. Gasparrini, TrAC Trends in Analytical Chemistry63, 95–103 (2014).
- D.C. Patel, M.F. Wahab, D.W. Armstrong, and Z.S. Breitbach, Journal of Chromatography A1467, 2–18 (2016).
- D.C. Patel, Z.S. Breitbach, J. Yu, K.A. Nguyen, and D.W. Armstrong, Analytica Chimica Acta963, 164–174 (2017).
- F. Gritti and G. Guiochon, Journal of Chromatography A1348, 87–96 (2014).
- D.C. Patel, Z.S. Breitbach, M.F. Wahab, C.L. Barhate, and D.W. Armstrong, Analytical Chemistry87, 9137–9148 (2015).
- O.H. Ismail, L. Pasti, A. Ciogli, C. Villani, J. Kocergin, S. Anderson, F. Gasparrini, A. Cavazzini, and M. Catani, Journal of Chromatography A1466, 96–104 (2016).
- S. Thurmann, C. Lotter, J.J. Heiland, B. Chankvetadze, and D. Belder, Analytical Chemistry 87, 5568–5576 (2015).
- L. Nováková and M. Douša, Analytica Chimica Acta 950, 199–210 (2017).
- D. Kotoni, A. Ciogli, I. D’Acquarica, J. Kocergin, T. Szczerba, H. Ritchie, C. Villani, and F. Gasparrini, Journal of Chromatography A1269, 226–241 (2012).
- C.L. Barhate, L.A. Joyce, A.A. Makarov, K. Zawatzky, F. Bernardoni, W.A. Schafer, D.W. Armstrong, C.J. Welch, and E.L. Regalado, Chemical Communications53, 509–512 (2017).
- M.F. Wahab, R.M. Wimalasinghe, Y. Wang, C.L. Barhate, D.C. Patel, and D.W. Armstrong, Analytical Chemistry88, 8821–8826 (2016).
- K. Fried and I.W. Wainer, Journal of Chromatography B: Biomedical Sciences and Applications689, 91–104 (1997).
- F. Ianni, R. Sardella, A. Lisanti, A. Gioiello, B.T. Cenci Goga, W. Lindner, and B. Natalini, Journal of Pharmaceutical and Biomedical Analysis116, 40–46 (2015).
- B. Kammerer, R. Kahlich, M. Ufer, S. Laufer, and C.H. Gleiter, Analytical Biochemistry339, 297–309 (2005).
- Y. Miyoshi, R. Koga, T. Oyama, H. Han, K. Ueno, K. Masuyama, Y. Itoh, and K. Hamase, Journal of Pharmaceutical and Biomedical Analysis69, 42–49 (2012).
- C.L. Barhate, E.L. Regalado, N.D. Contrella, J. Lee, J. Jo, A.A. Makarov, D.W. Armstrong, and C.J. Welch, Analytical Chemistry 89, 3545–3553 (2017).
- C. Lotter, E. Poehler, J.J. Heiland, L. Mauritz, and D. Belder, Lab on a Chip16, 4648–4652 (2016).
- D.R. Stoll and P.W. Carr, Analytical Chemistry89, 519–531 (2017).
- H. Gerhardt, A. Sievers-Engler, G. Jahanshah, Z. Pataj, F. Ianni, H. Gross, W. Lindner, and M. Lämmerhofer, Journal of Chromatography A 1428, 280–291 (2016).
Ulrich Woiwode is a PhD student under the supervision of Michael Lämmerhofer. He received his degree in pharmacy from the University of Tübingen, Germany, in 2013. His focus is on enantioselective separation techniques.
Stefan Neubauer received his Ph.D in biotechnology from the University of Natural Resources and Life Sciences, BOKU, Vienna, Austria, in 2012. After three years of postdoctoral work at the division of analytical chemistry at BOKU, under the supervision of Gunda Koellensperger and Stephan Hann, he joined the working group of Michael Lämmerhofer in Tübingen. He now works on impurity profiling via (2D-) LC in combination with high resolution mass spectrometry and complementary detection systems.
Mike Kaupert is a chemical technical assistant in the group of Michael Lämmerhofer. He has expertise in column packing, chromatographic separation, and various instrumental analysis methods.
Wolfgang Lindner is a Professor Emeritus of the University of Vienna, Austria, where he held the Chair of Analytical Chemistry of the Faculty of Chemistry. His research domain relates to pure and applied separation technologies, including mass spectrometry. He has a strong interest in research centres around the “power of selectivity”, including enantioselectivity and molecular recognition in LC and MS methodologies, which also leads to the developments of diverse stationary phases for LC. He has published ca. 500 articles and holds several diverse patents.
Michael Lämmerhofer is Professor for pharmaceutical (bio-)analysis at the Institute of Pharmaceutical Sciences at the University of Tübingen. His research focus spans pharmaceutical and biopharmaceuticals analysis, bioanalysis, and the development of functionalized materials. The development of enantioselective separations are a core expertise
in his group.

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









