Top-Down Mass Spectrometry of Intact Proteins and Complexes: From Rare to Routine
It has been technically feasible to transfer intact proteins and even noncovalent complexes into the gas phase for several decades now, and gas-phase fragmentation of the resulting ions was performed in the 1990s. For a long time, such approaches remained mostly academic curiosities; however, in recent years, there has been an “organic” evolution in instrument capabilities that resulted in deliberate initiatives that have enhanced access to such methods by a range of laboratories. Here, the recent trajectory of these methods from academic rarity to (near-) routine is briefly described, and an outlook about the future of these methods is provided.
The editors at Nature Methods named top-down mass spectrometry (MS) a “method to watch” in 2008 (1) because of the ability of this approach to fully characterize proteoforms, a term not coined until a few years later (2). It is remarkable how, in a fairly short period since then, top-down MS has matured to the point where top-down proteomics can be applied to complex samples on a fairly routine basis. This advancement can be largely attributed to the technological improvements made in all stages of analysis, including sample preparation, chromatography, high-performance MS, and data processing. Ultimately, however, it is important to keep in mind that differences between proteoforms are important primarily because they modulate intra- and intermolecular interactions in vivo and potentially have different downstream biological effects. To understand the mechanisms behind this modulation, proteins have to be studied close to their native context. MS is ideally suited for this type of study since native ionization allows for capturing a “snapshot” of an ensemble of different coexisting conformations and stoichiometries that are present in a solution, and top-down fragmentation subsequently allows dissecting these species, which has opened up new avenues for MS-based structural biology studies.
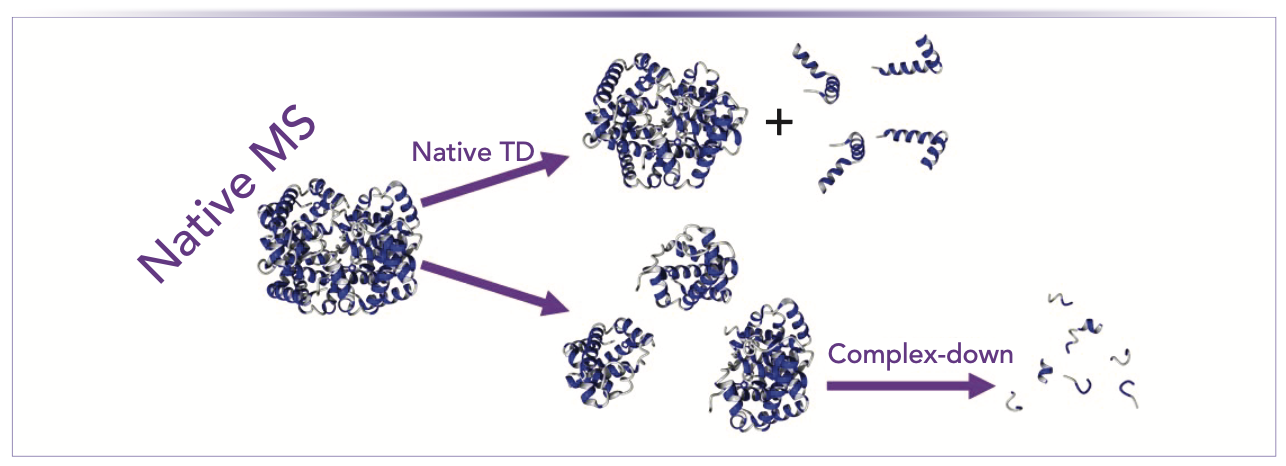
Two types of experiments can be used when combining native ionization with top-down fragmentation (3,4). The first experiment involves the investigation of noncovalent complexes by native ionization followed by the gas-phase ejection of monomers. Subsequently, the fragmentation of these monomers is evaluated, which allows elucidating the proteoform-specific stoichiometry of noncovalent complexes. In a previous study, the triosephosphate isomerase complex was analyzed, and the presence of three proteoforms was demonstrated—an unmodified one, an N-terminally acetylated one, and one that was phosphorylated at residue Ser20. It was shown that the phosphorylated and acetylated proteoforms do not dimerize with themselves or each other, indicating that these modifications regulate (specifically, inhibit) the formation of the dimer (5). Because a complex is broken down into its constituent monomers prior to extensive backbone fragmentation, this approach has been referred to as “complex-down MS” (3,4) This name was introduced to distinguish this approach from the second type of experiment, which combines native ionization with top-down fragmentation. This experiment, referred to as “native top-down,” aims primarily at obtaining information on the folding (secondary and tertiary structure) of proteins existing in the solution either as monomers or as subunits within complexes. It accomplishes this process by inducing (top-down) backbone cleavage within a native-like protein in the gas phase. The difference between complex-down and native top-down is illustrated schematically in Figure 1. One very early example was provided through collision-induced dissociation of the peptide RES-701-1 (6). This peptide has a “lasso” structure, and it was shown that the C-terminal end, which passes through the “ring”, is protected from fragmentation. This embedding of the C-terminus was not replicated in a synthetic version of the peptide, and abundant C-terminal fragmentation was observed in that analogue, showing that the higher-order structure modulated the fragmentation pattern. Nowadays, collision-based fragmentation is very rarely used in native top-down experiments, as phenomena such as (partial) unfolding and monomer ejection generally precede backbone fragmentation. Cases like the RES-701-1 peptide, where noncovalent interactions are so strong that they survive up to the internal energies required for backbone fragmentation, are the exception rather than the rule.
FIGURE 1: Illustration of native top-down and complex-down mass spectrometry (MS).
Most native top-down studies today rely on interaction of protein ions with electrons (electron capture or transfer dissociation) or ultraviolet photons (ultraviolet photodissociation) (4). Unlike collision-induced dissociation, these methods allow backbone fragmentation without significant disruption of noncovalent interactions. As such, the fragmentation pattern is modulated by the higher-order structure. Of course, the implication is that—in contrast to complex-down experiments—full sequence coverage is not the goal of the native top-down approach because the absence of fragments from particular sequence regions can be informative. Approximately 20 years ago, the electron capture dissociation spectra of different charge states of ubiquitin were compared, showing that low charge states primarily release fragments from the terminal regions, with the release of fragments resulting from backbone cleavage in the central region of the sequence being observed mainly with high charge state precursors (7). This behavior was attributed to the progressive unfolding of the structure by salt bridge disruption with increasing charge state. A similar pattern was observed with temperature- or laser-induced disruption of the protein structure. Moving away from peptides and small proteins, an interesting model system is the alcohol dehydrogenase tetramer, which has been investigated with all three native top-down methods mentioned above (electron capture dissociation (8) electron transfer dissociation (9,10), and ultraviolet (UV) photodissociation (11)). For all three methods, studies are available where partial collision-induced unfolding was followed by backbone fragmentation, and this structural disruption consistently led to a change in the fragmentation pattern, which demonstrates the potential of native top-down for the rapid probing of protein fold- ing and noncovalent interactions.
An interesting intermediate between the complex-down and native top-down approaches is the ejection of monomers or subcomplexes without (extensive) backbone fragmentation. Ejection of intact monomers is possible through carefully controlled collisional activation and can be helpful for determining the stoichiometry of a complex, because the mass of the complex and constituent subunits can be determined. This approach provides less detail than the complex-down workflow because detailed proteoform characterization is not performed. The ejection of subcomplexes is particularly interesting because it provides information on subunit connectivity within the original complex. Information on subunit connectivity in the original complex is usually not achieved through collisions with an inert gas because it requires deposition of sufficient energy to induce ejection of subcomplexes on a timescale that is too short to allow significant salt bridge rearrangement or monomer unfolding. Using a timescale that is too long typically results in the ejection of a highly charged monomer as exploited in complex-down experiments.
The best-characterized method for inducing the ejection of subcomplexes is surface-induced dissociation. In a recent example, the structure of the heterocomplex, Mnx, was investigated, and it was shown that it consists of a large (138 kDa) subunit connected to a heterohexamer made up of three 11.2 kDa and three 12.2 kDa subunits in an alternating sequence (12). As these approaches without extensive backbone fragmentation allow the virtual reconstruction (or “building up”) of the structure of a complex from the observation of subunits or subcomplexes, the name “complex-up MS” has been proposed for this type of experiment (3,4).
The coming years will see further adoption of the complex-up, complex-down, and native top-down approaches, as well as the expansion of the application field. The Consortium for Top-Down Proteomics has completed a number of initiatives to this end in recent years, including one on top- and middle-down characterization of monoclonal antibodies (mAbs) (13), one on the best practices for top-down MS (14), and one to define a standardized notation for proteoforms (15). The importance of the third aspect, and of broadly accepted standards for data handling in general should not be underestimated, because data processing and, in particular, confidence metrics for identification—including full or partial proteoform characterization—are important in allowing broad adoption of top-down proteomics (for example, in a clinical context). Consortium-led initiatives on combining native ionization with top-down analysis and using capillary electrophoresis for separating proteins and complexes prior to ionization are currently ongoing.
In addition to the deliberate push toward increasingly adopting these initiatives, there have recently been a number of exciting technological developments, particularly in regards to implementing advanced fragmentation methods on a range of mass analyzers. These technological developments allow greater experimental flexibility, particularly in combination with native ionization. For example, efficient electron capture dissociation through a commercially available instrument modification has been demonstrated on orbital trap MS (16,17) and quadrupole time-of-flight (QTOF) (18) instruments, with the latter also allowing the combination with ion mobility (IM) separation in a single measurement. UV photodissociation is also commercially available on certain orbital ion trap platforms, and recently surface-induced dissociation was launched commercially on an IM–mass spectrometer (MS) instrument. These are all high-end platforms, requiring significant capital investment and—for the time being—also significant user expertise. However, the fact that no in-house modification of instruments is required will significantly lower the barrier to entry into this field. These improvements will in turn pave the way for more widespread application of these methods, and will allow us to routinely start thinking about proteoforms in three dimensions.
References
(1) A. Doerr, Nat. Methods 5, 24 (2008).
(2) L.M. Smith and N.L. Kelleher, Nat. Methods 10, 186–187 (2013).
(3) F. Lermyte, Y.O. Tsybin, P.B. O’Connor, and J.A. Loo, J. Am. Soc. Mass Spectrom. 30, 1149–1157 (2019).
(4) M. Zhou, C. Lantz, K.A. Brown, Y. Ge, L. Pasa Tolic, J.A. Loo, and F. Lermyte, Chem. Sci. 11, 12918–12936 (2020).
(5) O.S. Skinner, N.A. Haverland, L. Fornelli, R.D. Melani, L.H.F. DoVale, H.S. Seckler, et al., Nat. Chem. Biol. 14, 36–41 (2018).
(6) J.A. Loo, J.X. He, and W.L. Cody, J. Am. Chem. Soc. 120, 4542–4543 (1998).
(7) K. Breuker, H. Oh, D.M. Horn, B.A. Cerda, and F.W. McLafferty, J. Am. Chem. Soc. 124, 6407–6420 (2002).
(8) H. Zhang, W. Cui, J. Wen, R.E. Blankenship, and M.L. Gross, Anal. Chem. 83, 5598–5606 (2011).
(9) F. Lermyte, A. Konijnenberg, J.P. Williams, J.M. Brown, D. Valkenborg, and F. Sobott, J. Am. Soc. Mass. Spectrom. 25, 343–350 (2014).
(10) F. Lermyte and F. Sobott, Proteomics 15, 2813–2822 (2015).
(11) M. Zhou, W. Liu, and J.B. Shaw, Anal. Chem. 92, 1788–1795 (2020).
(12) M. Zhou, J. Yan, C.A. Romano, B.M. Tebo, V.H. Wysocki, and L. Pasa-Tolic, J. Am. Soc. Mass. Spectrom. 29, 723–733 (2018).
(13) K. Srzentic, L. Fornelli, Y.O. Tsybin, J. A. Loo, H. Seckler, J.N. Agar, et al., J. Am. Soc. Mass. Spectrom. 31, 1783–1802 (2020).
(14) D.P. Donnelly, C.M. Rawlins, C.J. DeHart, L. Fornelli, L. F. Schachner, Z. Lin, et al., Nat. Methods 16, 587–594 (2019).
(15) R.D. LeDuc, V. Schwammle, M.R. Shortreed, A.J. Cesnik, S.K. Solntsev, J.B. Shaw, et al., J. Proteome. Res. 17, 1321–1325 (2018).
(16) J.B. Shaw, N. Malhan, Y.V. Vasil’ev, N.I. Lopez, A. Makarov, J.S. Beckman, and V.G. Voinov, Anal. Chem. 90, 10819–10827 (2018).
(17) M.A. den Boer, J.F. Greisch, S. Tamara, A. Bondt, and A.J.R. Heck, Chem. Sci. 11, 11886–11896 (2020).
(18) J.P. Williams, L.J. Morrison, J.M. Brown, J.S. Beckman, V.G. Voinov, and F. Lermyte, Anal. Chem. 92, 3674–3681 (2020).
Frederik Lermyte is with the Conformation-Sensitive Mass Spectrometry Laboratory in the Department of Chemistry at Technische Universität Darmstadt, in Darmstadt, Germany. Direct correspondence to: frederik.lermyte@tu-darmstadt.de.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.












