Mass Spectrometry Support to Mitigate Product Recall Situations in the Pharmaceutical Industry
In recent years, the pharmaceutical industry has been impacted by many significant product-related recalls, such as the 2,4,6-tribromoanisole (TBA)-related recall approximately 10 years ago, and, more recently, the nitrosamine for original and generic drug products, as well as benzene contamination issues in over-the-counter (OTC) consumer products. Those issues impacted the industry financially as well as tainted their reputation. The pharmaceutical industry initiated extensive investigations and implemented solutions to control and avoid those quality- and safety-related issues. In this article, case studies are presented to highlight the importance of mass spectrometry (MS)-based analytical solutions when extremely low detection limits are required, and when development resources are limited.
Product recalls in the pharmaceutical industry are mostly associated with either health or safety impacts to the patients, or quality-related issues. What is common in both cases is that the investigations to identify the root cause of a recall situation are complex, and they are outside the “routine operation frame” of the industry. Routine quality analysis for releasing pharmaceutical products to the market is still heavily based on analogue and nonselective detection chromatography techniques, such as gas chromatography–flame ionization detection (GC-FID) and liquid chromatography–ultraviolet diode-array detection (LC-UV/DAD). These detection methods have the benefit of relatively easy operation, a simple instrument qualification process, and straightforward analytical method validation. Such features are highly beneficial for routine quality control (QC) laboratories, and are sufficient for over 99% of the required applications. However, for a small, yet critical, percentage of cases, where an unknown peak shows up, these methods are not effective tools for performing appropriate investigations, and the retesting of the finished products with the methods are risky. In addition, there are recall situations when the issue is being identified by a sensory complaint, and the routine test methods are not capable of detecting the problem.
Mass spectrometry (MS) seems to be an ideal solution to handle those “unknown related situations,” because they can provide additional spectral information when an unusual peak is present, or, if used in the targeted detection mode, can detect specific targets at extremely low (ng/g or pg/g) levels. The highly selective detection modes of the mass spectrometer (specifically, selected reaction monitoring or high-resolution accurate mass ion extraction) are capable of drastically reducing the interferences associated with complex pharmaceutical matrices. The major roadblocks to widespread use of the MS-based technology in the routine operations are the capital cost of the instrument and the operation of the system because it requires highly trained analytical scientists.
This article presents two cases associated with recall situations. One of the recalls was related to a byproduct of a wood treatment chemical with an extremely low odor threshold. That case presented no known health risk to consumers, and was defined as a quality-related product recall. The more recent case is a safety recall related to benzene above the limit level in certain over-the-counter (OTC) consumer products.
Case I
Roughly a decade ago, a case of trace level migrant chemicals resulting from wood pallets caused a multibillion dollar impact on the pharmaceutical and food industries. Companies, such as General Mills from the food sector, as well as Pfizer and Johnson & Johnson from the pharmaceutical industry, recalled multiple products because of contamination by a degradation product of a brominated fire retardant used to treat wood pallets (1–3). Since the trace level migrant of 2,4,6-tribromoanisole has an extremely low sensory threshold, it can be easily detected by smell, and therefore it was relatively easy to identify its presence in the finished products. However, it was extremely difficult to detect and quantify this impurity under current good manufacturing practice (cGMP) conditions using a validated analytical method that had sensitivity close to the human olfactory threshold (4,5). The evaluation of these compounds in pharmaceutical formulations puts additional demands on method requirements. One of the most challenging aspects encountered in analyzing halophenol and haloanisole compounds in pharmaceutical formulations is the low detection limit required (about 10 pg/tablet or less for anisoles, and about 1 ng/tablet level for the phenols preferably) to match the detection limit of the human nose. The low level of detection requires eliminating any possible spectral interferences associated with finished pharmaceutical drug products and the siloxane-based gas chromatography (GC) column (see Figure 1).
FIGURE 1: (a) Isotope pattern for tribromo anisole molecular ion cluster, and (b) the isotope pattern of a common cyclic siloxane, present in a GC–MS background.
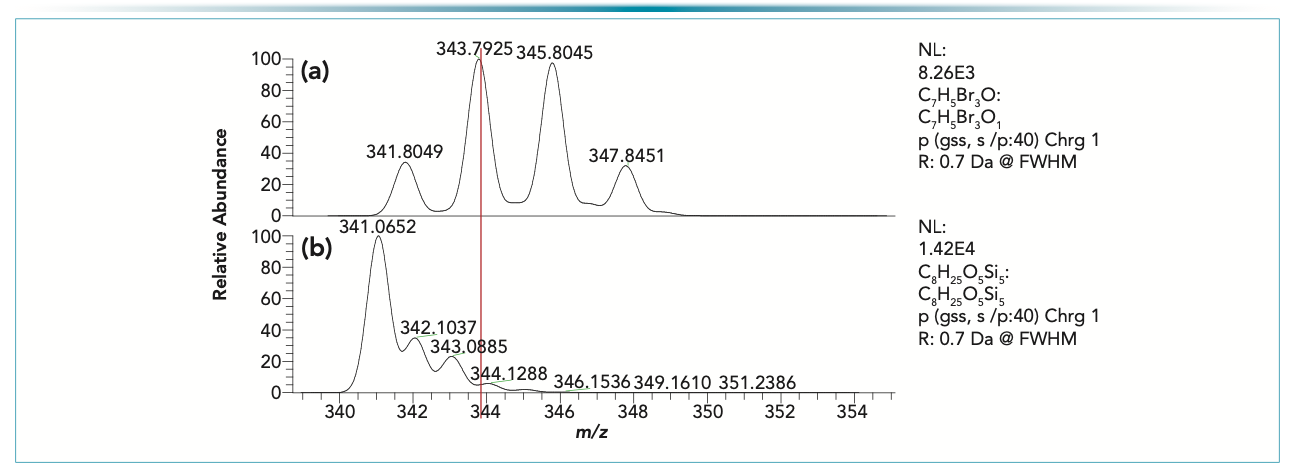
It is clear that if the target compound peak is at a lower concentration level than the interference peak, then the majority of the signal is associated with the background, resulting in a false positive test result. Eliminating the spectral interference leads to the development of a selected reaction monitoring (SRM). Since the peak with a nominal mass of m/z = 344 is associated with two different structures and elemental compositions (see Figure 1), the CID fragmentation is different. Therefore, a unique transition can be selected for specific and sensitive detection of the target analyte (see Figure 2).
FIGURE 2: SRM traces for 2,4,6 tribromo-anisole at a 20 fg/mL level using stir bar sorptive extraction. 15 mL sample was extracted, resulting in 300 fg of target analyte on-column; (a) quantifier transition, and (b) qualifier transition.
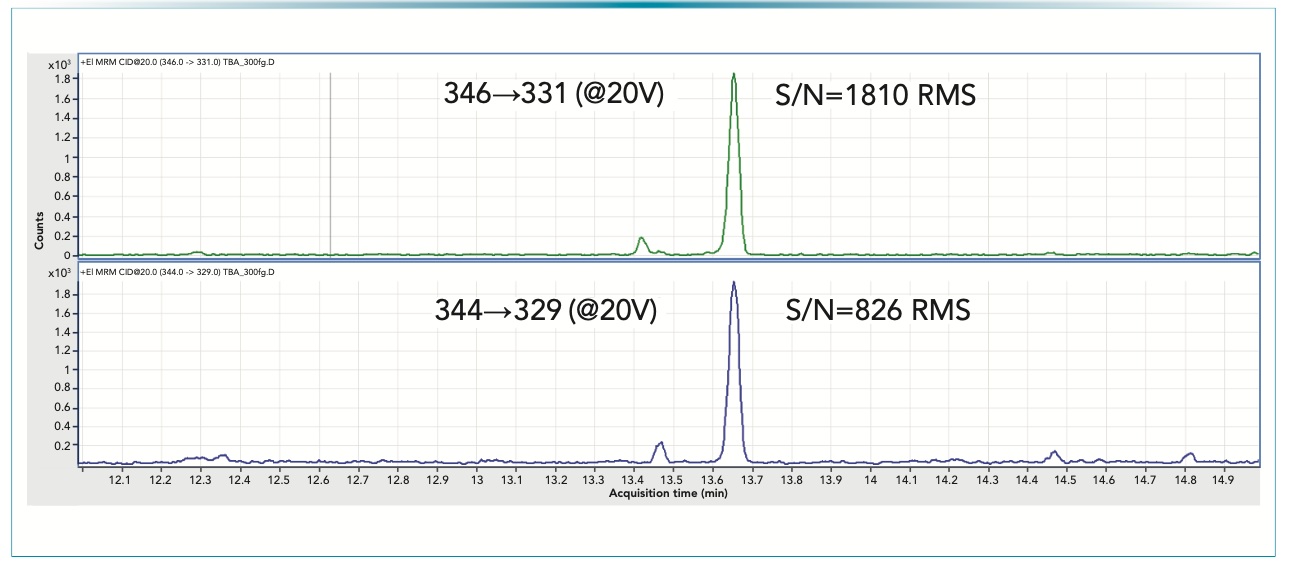
Such a low level detection requires a sample pretreatment to transfer a sufficient amount of the target compound to the GC–MS system. The most “popular” sample preparation option is based on solid phase microextraction (SPME), which was developed for trichloro-anisol (cork taint) in wine samples (4); however, the SPME option was successful only for the formulations with relatively “simple” excipient matrices. For more complex pharmaceutical formulations, a stir bar sorptive extraction based method was developed and validated to satisfy the request from the U.S. regulatory agency (5). The method was capable of detecting multiple haloanisols at 1–2 pg/tablet and halophenols at 70–95 pg/tablet levels, respectively, which has met the expectations of the regulatory agency set for 100 pg/tablet (5).
In summary, a GC tandem mass spectrometry (GC–MS/MS)-based technique was developed and implemented in a short period of time for the temporary release of finished pharmaceutical products.
Case II
Recent voluntary recalls of finished pharmaceutical products and consumer products (6,7) associated with benzene levels above the USP safety limits (8,9) highlight the importance of the analytical testing for toxic substances in finished pharmaceutical and consumer products. In both cases, the recalls were preceded by citizen petitions made by Valisure. The impacted products included hand sanitizers, primarily in gel form, and sunscreens, primarily in pressurized container packaging, using isobutane as the propellant. Benzene is a known carcinogen substance (10), and it is defined as Class I solvent in USP General Chapter <467> (8), and in the Food and Drug Administration (FDA) residual solvent guidance (11,12).
Residual solvents are classified into three classes based on risk assessment. Class 1 (residual solvents) represents “solvents to be avoided.” These solvents are known to be human carcinogens, or are strongly suspected human carcinogens. The USP chapter indicates “if (the solvent’s) use in order to produce an official product with a significant therapeutic advance is unavoidable, their levels should be restricted.” As benzene is not necessary for manufacture of hand sanitizer or sunscreen, it should not be present in the finished products. The FDA published a method for determination of benzene in hand sanitizer, and utilized the 2 ppm limit. To assign the associated risk for sunscreen, we need to understand the daily exposure level for benzene when the finished product is being used as intended. Approximately 28 grams/applications, and up to four applications/day (9), which results in a maximum daily use of 112 grams of the sunscreen, could result in a 224 μg/day benzene exposure if the products reach the limit set in the USP <467> standard. However, the USP chapter limits are intended for a maximum daily use of 10 grams of a finished product, consistent with the US FDA published permissible limit of 20 μg (0.02 mg)/day (13). Application of the 20 μg per day limit and the Option 2 approach listed in USP <467> for a dose of 112 grams result in a 0.18 ppm limit in sunscreen products (9).
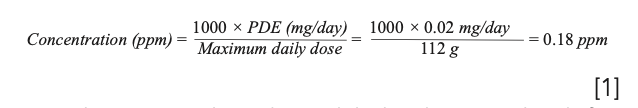
As the FDA already published a method for determining benzene in hand sanitizers, the focus was placed on developing a method appropriate for sunscreen and propellant used in sunscreen spray products, requiring a different sampling approach compared to the hand sanitizer. The analytical method is based on USP <467>, using static head space with flame ionization detector (FID) detection modified to an MS/ MS based detection, so as to achieve higher specificity and lower limit of detection.
The method employs a DB-624 capillary GC column with a dimension of 25 meter 0.2 mm internal diameter and 1.12 μm film thickness (β = 44.6) to provide separation of benzene from other volatiles. This column is different from the one listed in the USP <467>; however, the β value of the column is similar, therefore the chromatographic separation is expected to be the same or very similar, with the benefit of a lower carrier flow and shorter retention times associated with a shorter column length and the smaller column internal diameter. Identification of benzene is based on the retention time matching certified reference standards and mass spectral matching to benzene. Quantification of benzene is performed by comparing the peak area of benzene in a sample to a validated six-point calibration curve in a range of 20–800 ng/vial (equivalent of 0.1–4.0 ppm, assuming 200 mg of product weight).
The method in the USP <467> standard uses FID detection, appropriate for detecting and quantifying benzene, at or around the 2-ppm safety level. However, FID detection may not be sufficient for small sample sizes and lower levels of analysis (9). Another alternative is to use a single-stage MS-based detection that provides better selectivity and lower detection limit if an extracted ion trace specific for the benzene is being used. This approach requires a quantifier ion (for quantitation) and a qualifier ion (for confirming absence of spectral interferences).
To enhance the specificity of the detection method, and reduce the potential chemical and spectral interferences, an MS/MS method was developed for the detection. A transition of m/z = 78→52 was selected based on the product ion spectra. The test results using the two different detection methods for benzene for a compressed propellant sample, which contains benzene below the USP limit of 0.18 μg/g, are presented in Figure 3.
FIGURE 3: Chromatographic peak associated to a benzene peak present in a compressed propellant sample. The peaks represent 28 ng/vial. (a) MS/MS 72→52; (b) extracted ion chromatogram for m/z = 78 acquired in scan data acquisition.
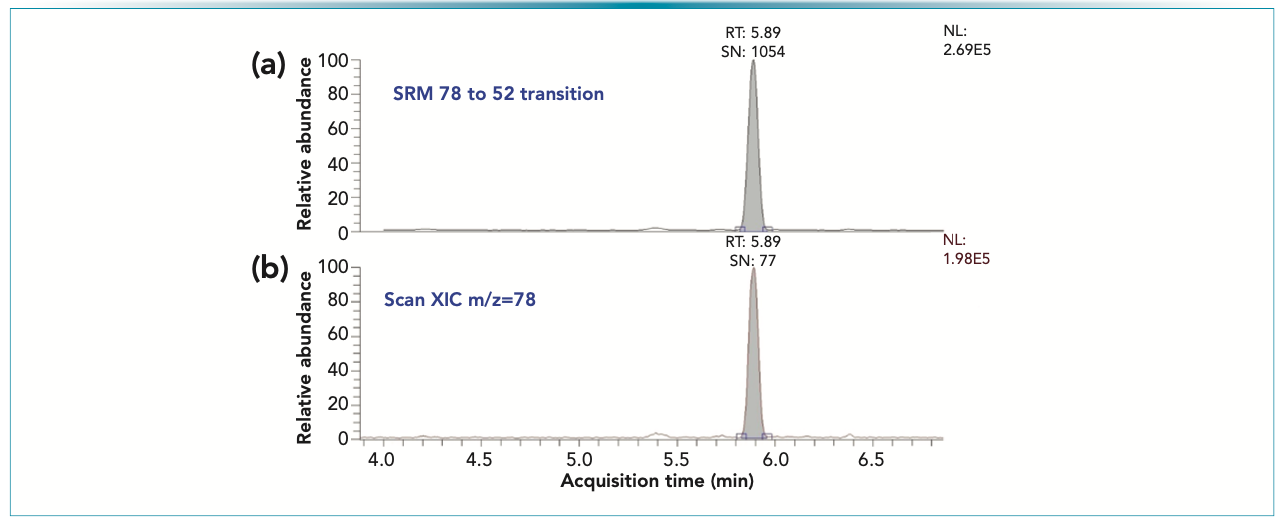
Both methods provided distinct chromatographic peaks, making the integration and quantitation straightforward. However, the peak in the MS/MS trace shows much higher signal-to-noise (S/N) value, and a little higher peak intensity compared to the extracted ion data (Figure 3). It is important to note that the S/N achieved for such a level of benzene is superior compared to the GC-FID based residual solvent method for Class I solvents (14,15).
In summary, a GC–MS/MS-based method was rapidly developed as a reliable alternative method for detecting trace level of benzene in compressed gas excipients. The mass spectrometry based method is more reliable to test samples with relatively high daily doses, where the GC-FID-based USP <467> method has serious limitations.
References
(1) A. Sawant, “Preliminary results of a PDA task force examining the cause, prevention and management of TBA and TCA taints,” from the 2011 PDA/ FDA Pharmaceutical Supply Chain Conference (June, 2011).
(2) SJN-DO WL Response McNeil Las Piedras PR 2-5-2010, source https:// www.fda.gov/media/78513/download
(3) G. Vas, L. Fleck, A. Michelson, N. Dunn, J. Duett, and J. Cali, Rev. Sep. Sci. 3(1), 3–20 (2021).
(4) T.J. Evans, C.E. Butzke, and S.E. Ebeler, J. Chromatogr. A 786, 293 (1997).
(5) J-T. Huang, L. Alquier, J.P. Kaisa, G. Reed, T. Gilmor, and G. Vas, J. Chromatogr. A 1262, 196–204 (2012).
(6) https://www.nbcnews.com/news/us-news/johnson-johnson-recalling-neutrogena-aveeno-sunscreens-due-benzene-traces-n1274004
(7) https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/ scentsational-soaps-candles-inc-issues-voluntary-nationwide-recall-scented-hand-sanitizers-due
(8) United States Pharmacopeia General Chapter <467> “Residual Solvents” (United States Pharmacopeial Convention, Rockville, Maryland).
(9) “Valisure Citizen Petition on Benzene in Sunscreen and After-sun Care Products,” and “Valisure Citizen Petition on Hand Sanitizer Products Containing Benzene Con- tamination and Other Significant Issues.” (2021)
(10) EPA Safety Risk Assessment for Benzene, https://www.epa.gov/sites/default/files/2016-09/documents/ benzene.pdf
(11) US Food and Drug Administration, Guidance for Industry: Residual Solvents in Drug Products Marketed in the United States (FDA, Rockville, Maryland, 2009).
(12) US Food and Drug Administration, Q3C—Tables and List Guidance for Industry (FDA, Rockville, Maryland, 2017).
(13) US Food and Drug Administration, Guidance for Residual Solvent Levels Q3C, Appendix 4 https://www.fda.gov/media/71738/download
(14) Agilent Technical Presentation USP <467> Residual Solvents Adapting to the New Requirements (2007).
(15) Practical Applications to USP <467> Implementation (SGS Life Sciences Technical Bulletin, 2009)
Gyorgy Vas is with VasAnalytical and Intertek Pharmaceutical Services, in Whitehouse Station, New Jersey. Direct correspondence to: gyorgy.vas@intertek.com
New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











