Extending the Reach: Non-Proximate Sampling for Mass Spectrometry Analysis of Large Objects and Surfaces
The ever-increasing sensitivity of commercial mass spectrometry (MS) instrumentation has enabled it to detect analyte ions generated at atmospheric pressure farther from the vacuum interface. However, if the object is too large or cannot be moved immediately adjacent to the instrument, ions pulled through atmospheric pressure transfer tubing become subject to egregious losses as the distance increases from centimeters to meters. Although a remote ion source and simple tube can be used, a truly capable analytical system requires a robust signal. Alternative models for direct, non-proximate sample analysis are neutral desorption and transfer of gas-phase analyte to an ion source/MS, condensed-phase extraction and transfer, and the extension of instrument vacuum to the sample.
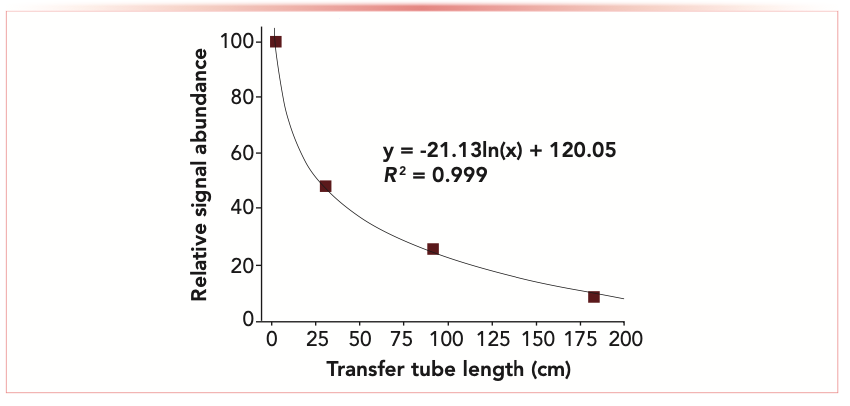
To paraphrase the poet Robert Browning, a mass spectrometer (MS)’s reach should exceed its grasp. The reverse is currently true—although MS instrumentation has become more adept at grasping ions at the atmosphere-to-vacuum transition using wide inlet orifices, ion lenses and guides, and ion funnels, less research has been devoted to reaching out to analytes on a surface at a distance from the MS instrument. Although ambient sampling and ionization methods continue to diversify, most systems position the ion source, analyte, and MS inlet within centimeters or less of one another. Many objects and surfaces are simply too large (or have other complicating circumstances) to be positioned immediately adjacent to the MS, necessitating some form of transfer between the atmospheric pressure desorption/ionization site and the instrument inlet. The length of the transfer tube effectively determines the largest surface dimension that can be accommodated. However, as the transfer tube length increases, ion transmission and analyte signal fall off logarithmically with distance as shown in Figure 1 and in prior literature (1).
FIGURE 1: Signal decrease from a vacuum-assisted analyte ion transmission with the length of the atmospheric pressure transfer tube between the post-plasma ion source and the MS inlet. (Newsome 2018, unpublished)
This practical limitation has caused method development to look toward onsite residual collection for later analysis (bringing vestiges of sample closer to the MS) or instrument miniaturization for portability (bringing the mass analyzer closer to sample). However, the former is discontinuous analysis of material related to a target, and the latter is rarely available and does not currently offer the capabilities of high-resolution laboratory instrumentation. For continuous, direct MS analysis, there are four models for non-proximate MS.
Atmospheric Pressure Ion Transfer
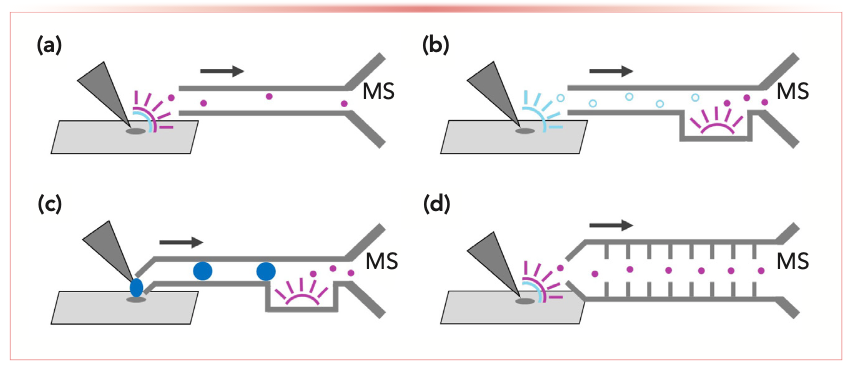
Regardless of the predictable signal loss at atmospheric pressure, directing analyte ions into a long, atmospheric pressure transfer tube (Figure 2a) is otherwise convenient, cheap, and “good enough” for some applications. Early successes in ambient ionization source design led the Cooks laboratory to test the limits of pulling ions through a stainless steel tube. At the cost of orders of magnitude in signal reduction, the tubing conducted desorption electrospray ionization (DESI) analytes from sample surfaces 3 m distant (2). The Fournier laboratory SpiderMass system later equaled that distance with flexible polytetrafluoroethylene (PTFE) tubing and a supplemental vacuum to sample from laser ablation/ionization of biological tissues (3). The Smithsonian Museum Conservation Institute interrogated a wooden object 30 cm from the MS instrument with direct analysis in real time (DART) (4). The Müller laboratory has more recently integrated spray-based and plasma-based ionization techniques into a handheld probe, using flexible 60 cm polyetheretherketone (PEEK) or fluorinated ethylene propylene (FEP) tubing (5).
FIGURE 2: Models for non-proximate surface sampling: (a) desorption and ionization at sample for atmospheric pressure ion transfer to the MS instrument; (b) neutral desorption into gas phase for transfer to MS-proximate ionization source; (c) extraction into condensed phase for transfer to MS-proximate ionization source; and (d) desorption and ionization at sample for reduced-pressure ion transfer to the MS instrument.
Gas-Phase Neutral Transfer
Notwithstanding the analytical success and cost savings that can be had transferring ions through air, even the high sensitivity of contemporary commercial MS instruments will inevitably be challenged by greater distances. A logical way to subvert this limitation is to desorb or ablate the analyte material from a distant surface without ionization, then transfer the neutral, gaseous material to an ion source proximate to the MS (Figure 2b). Perhaps the most visible example of this systemic revision is rapid evaporative ionization MS (REIMS). The first non-proximate analysis method to examine organic molecules in complex samples, REIMS was originally conceived as generating ions from tissue vaporization in electrosurgery before transporting the ions through heated, flexible PTFE tubing to an MS instrument (6). However, after the technology was commercialized by Waters, a solvent addition/ heated collision source was incorporated into the instrument vacuum interface to drive voltage-free inlet ionization of the remotely generated analyte aerosol (7). Recent publications with the commercial REIMS system have used laser ablation for non-proximate sampling of analyte from many different biomaterials (8). The Zhou laboratory built a nonproximate system not particular to an MS instrument manufacturer which uses solvent and an ultrasonic probe to desorb tissue aerosols, aspirates 70 cm through PTFE, and ionizes with heat and vacuum immediately external to the MS inlet (9).
Other surface-sampling systems desorb and transfer smaller amounts of material. The Vertes laboratory designed a laser ablation chamber to more efficiently transfer aerosolized analyte through 60 cm Tygon tubing to an MS-proximate electrospray emitter (10). The soft ionization by chemical reaction in transfer (SICRIT) ion source from Plasmion ionizes gas-phase analytes with an in-tube dielectric barrier discharge as they are pulled through by the instrument vacuum. Having sampled from laser-ablated surfaces 11 cm away (11), the instrument shows potential for sampling from greater distances, such as the Reynolds laboratory system for atmospheric pressure chemical ionization (APCI) of analytes desorbed 1 m away (12). Using longer rigid tubing, the Smithsonian is constructing a system to desorb analytes with a heated gas jet, transfer 2 m through an in-tube dopant permeator (13), and deliver the desorbed analytes to an atmospheric pressure photoionization (APPI) lamp (14). The Larriba-Andaluz laboratory is using computational fluid dynamics to design a more efficient inlet to collect such gaseous desorption products (15).
Condensed-Phase Transfer
Condensed-phase extraction is another model for neutral sampling. The most common form is liquid extraction surface analysis, in which microliters of the solvent are placed in direct contact with a sample surface to collect the analyte. Non-proximate sampling adaptations move the extract a significant distance—a grander scale than DESI or the Prosolia Flowprobe—before direct ionization and mass analysis (Figure 2c). The Eberlin laboratory developed the MasSpec Pen, a handheld interface used by a surgeon that creates a liquid micro-junction with biological tissue and then aspirates the droplet through 1.5 m PTFE tubing for inlet ionization (16). Similar systems have recently been demonstrated with analyses of more common objects. Oak Ridge National Laboratory created a handheld version of its open port sampling interface with liquid sample aspirated through 1 m of FEP tubing to an APCI source, successfully sampling from botanicals without peak broadening (17). Rather than transporting liquid droplets with the vacuum, a system constructed by the Spengler laboratory circulates extraction solvent through supply and return tubing between a handheld probe and a 50 cm-distant electrospray emitter (18). In addition to coupling to high-resolution instrumentation, the latter assembly was used with a miniature rectilinear ion trap to analyze various irregularly shaped biological and synthetic objects. A probe designed by the Chen laboratory circulates silk suture string instead of liquid over a 2 m path, recovering sample residue from biological tissue for ionization with MS-proximate electrospray droplets (19). Each of the various neutral-transport systems may be constructed in an academic setting with some effort and tolerance for complexity.
MS Vacuum Extension for Ion Transfer
With atmospheric pressure transfer so inefficient or complex, one can “stretch out” the MS with front-end mobility devices to transport ions from a distant sample. Arguably, the most technologically sophisticated solution to ion loss over transfer distance has been realized with SPion, developed by Trace Matters. The SPion components together are operated fundamentally as an extension of a commercial mass spectrometer vacuum interface (Figure 2d), using a flexible, 1.2 m-long stacked-ring ion guide installed in place of standard fore-vacuum optics to transport analyte ions with performance similar to a factory inlet (20). Unlike other non-proximate sampling systems, a particular ion source is not intrinsically necessary to the function of an ultralong, lossless inlet. The SPion could, therefore, be used for large molecule analysis or with DESI, DART, and other third-party ambient ion sources if it can be fitted with supplemental evacuation as necessary. A similar system cannot be as easily constructed by laboratory tinkerers and will not be so affordable, but it opens exciting possibilities to those who can get it.
Future Directions
The analyst who wishes to perform non-proximate sampling will have to choose instrumentation based on the limitations of budget and the requirements of the analyte—to invest in a vendor-exclusive system, a commercial but vendor-neutral system, or the time cost to build or modify a custom system. The deci- sion may also affect whether the MS instrument can be devoted entirely to non-proximate sampling or must be modular. The analyte object will impose requirements based on its size and tolerance for destructive sampling or solvent exposure, in addition to typical ambient analysis considerations like ionization type and potential for carry-over.
Whether the required scale is 20 cm, 2 m, or more, non-proximate sampling technology will be increasingly valuable. Even when portable, high-resolution instrumentation becomes available, it will require an effective transfer pathway between the atmospheric pressure sampling point and the mass analyzer. Most of the systems discussed here use a handheld probe, which can be moved to access irregular shapes and offers intuitive user appeal to make it effective in exotic settings like the operat- ing room and the industrial processing line. With efficient analyte transfer methodology, the ambient sampling mechanism need only be reproducible to make non-proximate analysis useful for stationary or mobile instruments in the laboratory and field.
References
(1) A. Kiontke, F. Holzer, D. Belder, and C. Birkemeyer, Anal. Bioanal. Chem. 410, 3715–3722 (2018).
(2) I. Cotte-Rodriguez and R.G. Cooks, Chem. Commun. 28, 2968–2970 (2006).
(3) B. Fatou, P. Saudemont, E. Leblanc, D. Vinatier, V. Mesdag, M. Wisztorski, C. Focsa, M. Salzet, M. Ziskind, and I. Fournier, Sci. Rep-UK 6, 1–14 (2016).
(4) T.P. Cleland, G.A. Newsome, and R.E. Hollinger, Analyst 144, 7437–7446 (2019).
(5) C. Meisenbichler, F. Kluibenschedl, and T. Muller, Anal. Chem. 92, 14314–14318 (2020).
(6) K.C. Schafer, J. Denes, K. Albrecht, T. Szaniszlo, J. Balog, R. Skoumal, M. Katona, M. Toth, L. Balogh, and Z. Takats, Angew. Chem. Int. Edit. 48, 8240–8242 (2009).
(7) E.A. Jones, D. Simon, T. Karancsi, J. Balog, S.D. Pringle, and Z. Takats, Anal. Chem. 91, 9784–9791 (2019).
(8) V. Plekhova, L. Van Meulebroek, M. De Graeve, A. Perdones-Montero, M. De Spiegeleer, E. De Paepe, E. Van de Walle, Z. Takats, S.J. Cameron, and L. Vanhaecke, Nat. Protoc. 2, 1–28 (2021).
(9) X. Fu, Y. Wang, B. Xia, P. Y. Shi, and Y. Zhou, Anal. Chem. 93, 10502–10510 (2021).
(10) J.A. Fincher, A.R. Korte, B. Reschke, N. J. Morris, M.J. Powell, and A. Vertes, Analyst 142, 3157–3164 (2017).
(11) S.K.I. Funke, V.A. Bruckel, M. Weber, E. Lutzen, J.C. Wolf, C. Haisch, and U. Karst, Anal. Chim. Acta 1177, 338770 (2021).
(12) S. Rankin-Turner, M.A. Turner, P.F. Kelly, R.S.P. King, and J.C. Reynolds, Chem. Sci. 10, 1064–1069 (2019).
(13) G.A. Newsome and T.P. Cleland, Anal. Chem. in press, https://doi.org/10.1021/acs.analchem.1c02400 (2021).
(14) G.A. Newsome, “Dopant-Assisted Desorption Atmospheric Pressure Photoionization Without a Solvent Jet for Non-Proximate Sampling,” presented at 69th ASMS Conference on Mass Spectrometry and Allied Topics, Philadelphia, PA, 2021.
(15) X. Chen, M.G. Buchanan, J. Glasper, G.A. Newsome, and C. Larriba Andaluz, “Flow Optimization of an Ambient Desorption Source for Solvent-free Analysis of Non-proximate Objects,” presented at 69th ASMS Conference on Mass Spectrometry and Allied Topics, Philadelphia, PA, 2021.
(16) J.L. Zhang, J. Rector, J.Q. Lin, J.H. Young, M. Sans, N. Katta, et al., Sci. Transl. Med. 9, 406 (2017).
(17) C.L. Walton, V. Kertesz, and J.F. Cahill, J. Am. Soc. Mass Spectr. 32, 198–205 (2021).
(18) F. Lotz, P. Baar, B. Spengler, and S. Schulz, Analyst 146, 3004–3015 (2021).
(19) L.C. Chen, T. Iwano, S. Ninomiya, T. Koike, Y. Tanaka, and K. Yoshimura, J. Am. Soc. Mass Spectr. 32, 606–610 (2021).
(20) M. Taghioskoui, https://www.tracematters.com/technicalnotes (2021).
G. Asher Newsome is an analytical chemist with the Smithsonian Museum Conservation Institute in Suitland, Maryland. Direct correspondence to: newsomeg@si.edu.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.












