In-Depth Analysis of Host Cell Protein (HCP) Impurities by LC–MS/MS to Augment Routine HCP-ELISA Testing of Biotherapeutics
The enzyme-linked immunosorbent assay (ELISA) is the industry standard for quantitative host cell protein (HCP) analysis in biopharmaceutical product and process development. HCP-ELISA affords total HCP measurements (ng/mg) with broad HCP coverage in a straightforward and reliable analysis format. HCP-ELISA supports batch release testing of bulk drug substance, as well as step-by-step confirmation of downstream process performance. Failure to remove residual HCPs sufficiently during downstream processing can potentially affect the quality, safety, and efficacy of the biotherapeutic. In recent years, liquid chromatography–tandem mass spectrometry (LC–MS/MS) has gained momentum as an orthogonal approach to HCP-ELISA. LC–MS/MS provides identification and quantitation of individual HCPs, and helps ensure that no HCPs evade HCP-ELISA detection above a reportable limit (for example, 10 ng/mg). The individual HCP information from LC–MS/MS is used to facilitate a multi-departmental risk assessment if one or more residual HCPs are present in final drug substance above 10 ng/mg.
Host cell proteins (HCPs) are residual protein impurities expressed along with the desired recombinant biotherapeutic product (1,2). Removing HCPs in the final drug substance (DS) to an acceptable level sometimes can be challenging because of the diverse nature of the HCPs and their potential affinity for the intended product (3). The lack of an affinity capture step in the purification train of a biotherapeutic product can make HCP removal more demanding. Additionally, the presence of potent HCP enzymes at trace level (<1 ng/mg), such as cathepsins (4,5) and lipases (6–8), can selectively cleave the polypeptide chain or degrade surfactants in the formulation buffer during long-term storage, respectively, creating an inconsistent product. Failure to remove residual HCPs sufficiently during downstream processing can affect biotherapeutic product quality, safety, and efficacy, or even induce adverse effects to patients (9–13). Therefore, HCPs are considered an obligatory critical quality attribute (CQA) that should be minimized throughout the biotherapeutic development cycle (14). Although there is no specific guidance from health authorities regarding acceptable limits for HCPs in the final drug product (DP), ICH Q6B (15), and ICH Q11 (16) affirm that HCPs must be closely monitored and reduced to acceptable levels.
Biopharmaceutical companies often utilize an enzyme-linked immunosorbent assay (HCP-ELISA) to monitor levels of total HCP at each step during downstream process and in final DS (17,18). HCP-ELISA is currently considered the gold standard for efficient HCP analysis because it affords broad coverage, high sensitivity, automation capability, and reliable quantitation in a rapid manner (2,12,14). Other analytical technologies, including gel electrophoresis and western blotting, can be utilized for HCP analysis, but they require more technical expertise and are considered orthogonal methods (19,20). Since 2012, many biopharmaceutical product and process development laboratories are applying a bottom-up proteomics strategy for HCP analysis via liquid chromatography–tandem mass spectrometry (LC–MS/MS) to augment HCP-ELISA (21–27). Here, LC–MS/MS provides identification and quantitation of individual HCPs with high sensitivity (>10 ng/mg). In this article, we review the standard LC–MS/MS workflow and demonstrate how the technique is applied as an effective orthogonal approach to HCP-ELISA during bioprocess development.
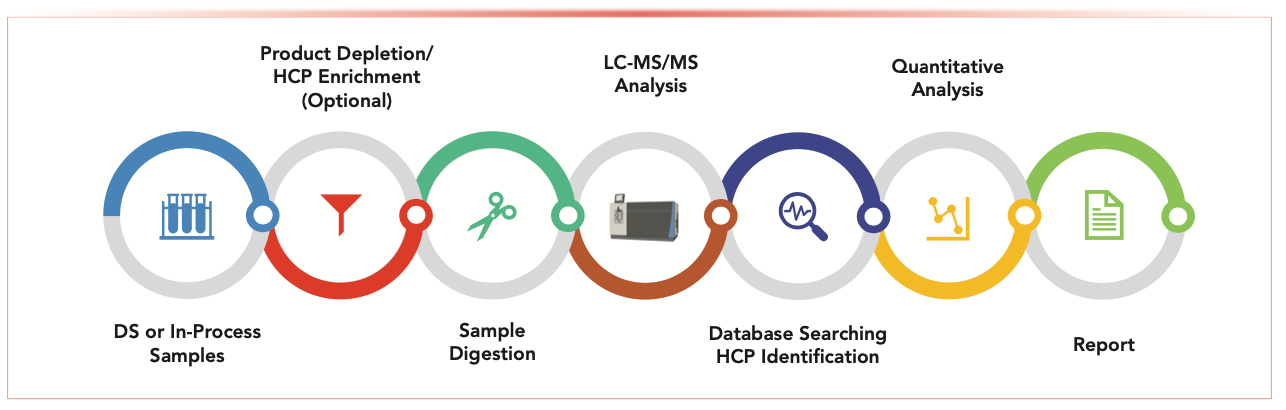
The typical LC–MS/MS workflow for HCP analysis is illustrated in Figure 1. Samples (either final DS or in-process samples) are digested by specific enzymes, such as trypsin or Lys-C. The resulting peptides from potential HCPs and the intended product are separated by reversed-phase LC (RPLC) and subsequently detected by an ultrahigh resolution, accurate mass research grade mass spectrometer. A majority of these peptides are fragmented by collisional activation in the gas phase to yield amino acid sequence information, which is used to identify both HCP- and product-related peptides through protein database searching. The protein databases are set up to contain the proteome of the host organism, sequence of the intended product, proteases, and common contaminants. Additionally, known problematic HCPs (28) should be included as a separate database to enhance confidence scores during database searching, especially since these HCPs are typically present at low ng/mg levels. Each HCP should be identified with at least two unique peptides. Acceptable peptide identifications include <1% false discovery rate (FDR) for a stringent search and <2–5% FDR for a less stringent search. Typically, individual HCPs above the quantitation limit (QL) of 10 ng/mg (if present) are reported for the final DS. For in-process samples, many HCPs will be observed prior to downstream processing with exponentially less and less HCPs after the affinity capture and polishing steps. Therefore, in addition to tabulating the extensive list of individual HCP identifications and relative abundances for each process step, it is equally powerful to report the number of HCPs before and after each purification step to demonstrate clearance, as well as use Venn diagrams to compare pre- and post-change samples to evaluate the effectiveness of process improvements in demonstrating product comparability.
FIGURE 1: A typical LC–MS/MS-based proteomics workflow for host cell protein (HCP) analysis.
HCP quantitation via LC–MS/MS is achieved on either a relative or absolute basis. Relative quantitation is often performed by the “top three” label-free method, where the summed peak areas for the three most abundant peptides from each HCP are compared against the summed peak areas of the three most abundant peptides obtained from either the biotherapeutic product or spiked-in protein standards. Both data dependent acquisition (DDA) (21,24,27) and data independent acquisition (DIA) (23,26,29,30) modes can be used. DDA is most often applied, whereas DIA offers better sensitivity for low abundant HCPs (since these peptides may be missed by DDA). However, confidence scores could be negatively impacted for coeluted peptides in DIA. Building a spectral ion library using in- process samples can be implemented to solve the issue, but the process is typically laborious, and the library needs to be updated when manufacturing process improvements are made (26). Conversely, for absolute quantitation, heavy-isotope-labeled peptide analogues from select HCPs can be spiked into the samples at known levels. Targeted proteomics tools, such as multiple reaction monitoring (MRM), parallel reaction monitoring (PRM), and selected reaction monitoring (SRM), can be applied for quantitative measurements (22). Additionally, the accuracy of both relative and absolute quantitation can be improved by applying either external or internal calibration curves based on spiked-in standards (13,26). Therefore, it is possible to obtain a summed value of all HCPs present via LC–MS/MS and compare trends of various samples with HCP-ELISA results. However, analysts need to be cautious in drawing conclusions because detection principles for HCP-ELISA and LC–MS/MS are fundamentally different. On the other hand, correlation of LC–MS/ MS and HCP-specific ELISA results for a particular HCP can usually be established (12,27). In summary, LC–MS/MS offers opportunities to obtain identification and quantitation of individual HCPs down to low ng/mg levels in a single experiment, which ensures development of a robust and well-controlled manufacturing process and enables enhanced process understanding.
Because the purified product is usually significantly more abundant than individual HCPs, as is the case for monoclonal antibodies (mAbs), HCP analysis challenges not only the sensitivity of the mass spectrometer but also the dynamic range. Multiple strategies, such as product depletion or HCP enrichment, can be applied to improve HCP detection (7,31). For example, some commonly used technologies, including molecular weight (MW) cutoff filters (32), LC fractionation (33,34), electrophoresis (35), and 2D sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) (36), effectively separate HCPs from the intended product based on the physiochemical properties of proteins, such as size, hydrophobicity, and isoelectric point. In addition, mAbs can be depleted effectively via a digestion protocol under native condition (37). On the contrary, HCPs can be enriched by affinity capture (7) or substrate binding (6,8). However, all depletion or enrichment approaches could potentially miss HCPs that bind to the intended product or have similar physiochemical properties as the product, as well as introduce additional variables for accurate quantitation. Alternatively, proteolytic peptides from HCPs can be analyzed by coupling two-dimensional LC with tandem MS (2D-LC–MS/MS) to further improve sensitivity (22,23,38). Furthermore, analysts can also apply nano-LC to improve sensitivity, although it is possible to sacrifice robustness and throughput with this approach (23).
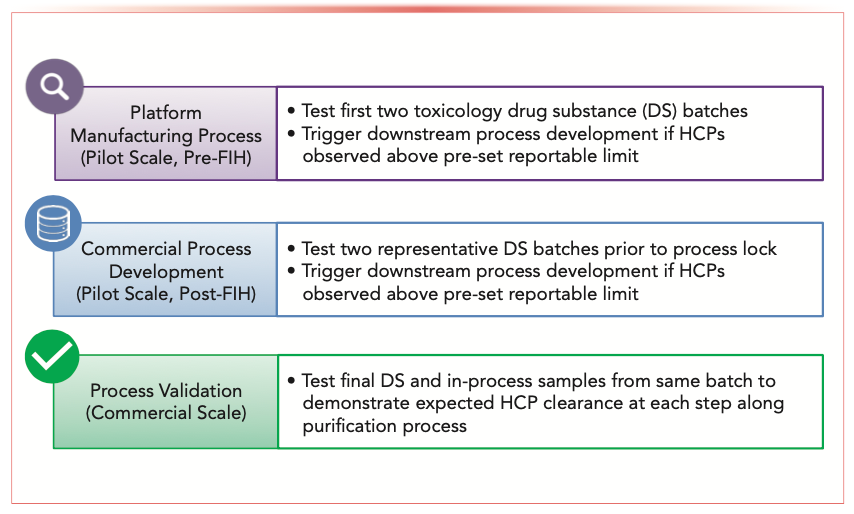
In practice, HCP-ELISA is used routinely for in-process sample analysis and DS batch release in quality control laboratories. HCP-ELISA provides evidence for HCP clearance along purification train and confirmation of a high-quality DS. However, one of the known limitations of HCP-ELISA is that the polyclonal antibodies (generated from immunization of animals by null cell line lysate) often have no or limited coverage to certain HCPs that are either non- or weakly immunogenic, leading to under-representation of these HCPs in the final readout. Therefore, LC–MS/MS can be applied to fill this gap during different stages of bioprocess development (Figure 2). At an early stage prior to start of clinical trials, LC–MS/MS analysis is used to verify that HCP-ELISA does not miss any HCPs in the pilot-scale DS at more than 10 ng/mg (a common reportable limit for HCPs across the industry). During subsequent commercial process development, several iterations of LC–MS/MS experiments are applied to ensure HCPs are effectively removed in the final pilot-scale DS before the process is locked (finalized), especially for those products without an affinity purification step. Upon manufacturing process validation, LC–MS/MS analysis of both the DS and in-process samples from the same batch is used to provide confirmation of HCP clearance. At any stage of the product development lifecycle, LC–MS/MS results enhance process understanding and help the bioprocess team develop and optimize the upstream and downstream manufacturing process conditions to minimize HCP production or optimize HCP removal. In addition, LC–MS/MS can be applied to enhance understanding of ELISA critical reagent coverage (39,40) to facilitate the periodic bridging activity between old and new critical reagents. More importantly, LC–MS/MS enables the required knowledge-based risk assessment to allow decision making during process development and discussions with regulatory agencies around control strategies (19,41). When higher level HCPs are observed during manufacturing process development, LC–MS/MS results can clarify if it is because of the emergence of new HCPs or increased level of existing HCPs. This practice is important because information on existing HCPs could be potentially correlated to historical toxicology or clinical experiences. On the contrary, lower level HCPs observed in a new batch by HCP-ELISA is not necessarily an indication of lower risk because of potential existence of problematic HCPs, which need to be considered during risk assessment.
FIGURE 2: Characterization roadmap for HCP analysis by LC–MS/MS to facilitate early and late-stage bioprocess development (FIH: first in human).
To summarize, one of the advantages of LC–MS/MS is that it allows direct measurements of individual HCPs in the final DS and throughout the purification process. Knowledge of individual HCPs in DS is critical for biotherapeutic manufacturers to ensure quality, safety, and efficacy of the medicine. Therefore, LC–MS/MS has gradually become an important orthogonal approach to HCP-ELISA, in addition to supporting in-depth process characterization and control. More importantly, LC–MS/MS could potentially reveal HCPs that are under-represented in HCP-ELISA and facilitate potential risk assessments, ensuring a comprehensive analytical characterization and process development data package for regulatory submissions. It is recommended to qualify the LC–MS/MS protocol for HCP analysis to ensure suitable method performance, confidence, and applicability to future projects and process improvement situations. To date, reliably detecting individual HCPs at <1 ng/mg levels remains challenging by LC–MS/MS, but work is ongoing at different biopharmaceutical companies and vendor partners to develop methods and instrumentation with better sensitivity, selectivity, and robustness (28). Hopefully, future technological advances will provide real-time bioprocess development support for HCP analysis and more.
References
(1) M. Vanderlaan et al., Biotechnol Prog. 34, 828–837 (2018).
(2) X. Wang, A.K. Hunter, and N.M. Mozier, Biotechnol. Bioeng. 103, 446–458 (2009).
(3) A.A. Shukla and P. Hinckley, Biotechnol. Prog. 24, 1115–1121 (2008).
(4) J.S. Bee et al., Biotechnol. Prog. 31, 1360–1369 (2015).
(5) H. Luo et al., Biotechnol. Prog. 35, e2732 (2019).
(6) X. Li et al., Anal. Chem. 93, 8161–8169 (2021).
(7) T. Graf et al., J. Pharm. Sci. S0022–3549(21)00353-1 (2021).
(8) S. Zhang, H. Xiao, R. Molden, H. Qiu, and N. Li, J. Pharm. Sci. 109, 3300–3307 (2020).
(9) M. Pavlovic, E. Girardin, L. Kapetanovic, K. Ho, and J.H. Trouvin, Horm. Res. 69, 14–21 (2008).
(10) R. Beatson et al., Biotechnol. Bioeng. 108, 2759–2764 (2011).
(11) S.X. Gao et al., Biotechnol. Bioeng. 108, 977–982 (2011).
(12) M. Vanderlaan, W. Sandoval, P. Liu, J. Nishihara, G. Tsui, M. Lin, et al., Bioprocess Int. 13, 18–22 (2015).
(13) X. Li et al., Biotechnol. Prog. 37, e3128 (2021).
(14) General Chapter <1132> “Residual Host Cell Protein Measurement in Biopharmaceuticals”, in United States Pharmacopeial 39-National Formulary 34 (United States Pharmacopeial Convention, Rockville, MD, 2016).
(15) International Conference on Harmonization, ICH Q6B, Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products (ICH, Geneva, Switzerland, 1999).
(16) International Conference on Harmonization, ICH Q11, Development and Manufacture of Drug Substances (ICH, Geneva, Switzerland, 2013).
(17) A.A. Shukla et al., Biotechnol. Prog. 24, 615–622 (2008).
(18) A.L. Tscheliessnig, J. Konrath, R. Bates, and A. Jungbauer, Biotechnol. J. 8, 655–670 (2013).
(19) M. Jin, N. Szapiel, J. Zhang, J. Hickey, and S. Ghose, Biotechnol. Bioeng. 105, 306–316 (2010).
(20) J. Zhu-Shimoni et al., Biotechnol. Bioeng. 111, 2367–2379 (2014).
(21) A. Michalski, J. Cox, and M. Mann, J. Proteome. Res. 10, 1785–1793 (2011).
(22) C.E. Doneanu et al., MAbs 4, 24–44 (2012).
(23) M.R. Schenauer, G.C. Flynn, and A.M. Goetze, Anal. Biochem. 428, 150–157 (2012).
(24) V. Reisinger, H. Toll, R.E. Mayer, J. Visser, and F. Wolschin, Anal. Biochem. 463, 1–6 (2014).
(25) D. Krawitz, J.C. Rouse, J.B. Sperry, W. Sandoval, and M. Vanderlaan, in Analytical Characterization of Biotherapeutics, J.R. Lill and W. Sandoval, Ed. (John Wiley & Sons, Inc., Hoboken, New Jersey, 2017), pp. 211–234.
(26) D.E. Walker et al., MAbs 9, 654–663 (2017).
(27) Y. Zhang, P. Baldus, N. Mozier, and J.C. Rouse, in Biopharmaceutical Emerging Best Practices Association (BEBPA) 5th Annual Host Cell Protein Conference: Best Practices for Immunoassay & LC–MS/MS Assessments of Product Purity (San Francisco, California, 2017).
(28) M. Jones et al., Biotechnol. Bioeng. 118, 2870–2885 (2021).
(29) Q. Zhang et al., MAbs 6, 659–670 (2014).
(30) C.E. Doneanu et al., Anal. Chem. 87, 10283–10291 (2015).
(31) K.N. Valente, N.E. Levy, K.H. Lee, and A.M. Lenhoff, Curr. Opin. Biotechnol. 53, 144–150 (2018).
(32) I.H. Chen, H. Xiao, T. Daly, N. Li, Anal. Chem. 92, 3751–3757 (2020).
(33) Q. Wang et al., Anal. Chem. 92, 10327–10335 (2020).
(34) M.P. Washburn, D. Wolters, and J.R. Yates III, Nat. Biotechnol. 19, 242–247 (2001).
(35) G. Zhu et al., Electrophoresis 35, 1448–1452 (2014).
(36) F. Fortis et al., J. Proteome. Res. 5, 2577–2585 (2006).
(37) L. Huang et al., Anal. Chem. 89, 5436–5444 (2017).
(38) A. Farrell et al., Anal. Chem. 87, 9186–9193 (2015).
(39) S.M. Henry, E. Sutlief, O. Salas- Solano, J. Valliere-Douglass, MAbs 9, 1065–1075 (2017).
(40) D.M. Waldera-Lupa et al., MAbs 13, 1955432 (2021).
(41) C.L. de Zafra, V. Quarmby, K. Fran- cissen, M. Vanderlaan, and J. Zhu-Shimoni, Biotechnol. Bioeng. 112, 2284–2291 (2015).
Ying Zhang and Jason C. Rouse are with Pfizer Inc., in Andover, Massachusetts. Direct correspondence to: ying.zhang06@pfizer.com.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









