Top 10 Considerations for Persistent Organic Pollutants Analysis by Gas Chromatography
Persistent organic pollutants (POPs) analysis by gas chromatography (GC) presents multiple challenges including critical pair resolution, temperature stability, reproducibility, and sensitivity issues, to mention a few. This article expands beyond the basic POPs analysis by GC to provide the top 10 aspects that need to be considered to obtain reliable chromatographic results.
Persistent organic pollutants (POPs) are toxic chemicals that adversely affect human health and the environment around the world. Most POPs generated in a single geographic location travel by wind and/or water to affect wildlife, human, and aquatic life far from where they are used and released. They persist in the environment for long periods of time due to the inherent stability in their structure, and accumulate in species and then are passed through the food chain. There is therefore a need to monitor these contaminants both in environmental and food samples. To address this global concern, the United States joined 90 other countries and the European community to sign a groundbreaking United Nations treaty in Stockholm, Sweden, in May 2001. Under the treaty, known as the Stockholm Convention, countries agreed to reduce or eliminate the production, use, and/or release of 12 key POPs. The “Dirty Dozen” includes some that are intentionally produced chemicals (aldrin, chlordane, dichlorodiphenyl trichloroethane [DDT], dieldrin, endrin, heptachlor, mirex, toxaphene), some that are unintentionally produced contaminants like polychlorinated dibenzo-p-dioxins(dioxins) and polychlorinated dibenzofurans (furans), and a few compounds that may be intentionally or unintentionally produced, including hexachlorobenzene and polychlorinated biphenyls (PCBs) (1). Aside from these, there are several emerging contaminants that get added to the list periodically. The compounds are often also organized by instrumentation or technique used for analysis and are classified into POPs that are analyzed by gas chromatography (GC) or liquid chromatography (LC). In this article, we will explore various aspects that need to be considered for GC POPs analysis.
1. Stationary Phase Selectivity for Resolution Improvement
Resolution improvement in chromatography can be influenced by three different factors: efficiency term, selectivity term, and retention term, as shown in Figure 1. Out of these three terms, selectivity is the most influential term to bring drastic improvement in resolution, which is presented in the following two examples. In the first example, polycyclic aromatic hydrocarbons (PAHs), a class of POPs that has multiple critical pairs (2), were analyzed on three different stationary phase selectivities that were designed for PAH analysis (Figure 2). The chromatogram demonstrates that, even though all three columns are of the exact same length and inner diameter, the change in stationary phase selectivity can bring in enhanced resolution. Hence optimal selectivity is the key to resolution improvement. In addition, stationary phase selectivity that can recognize small differences in the analyte can bring in shorter run times in addition to resolution improvement (3).
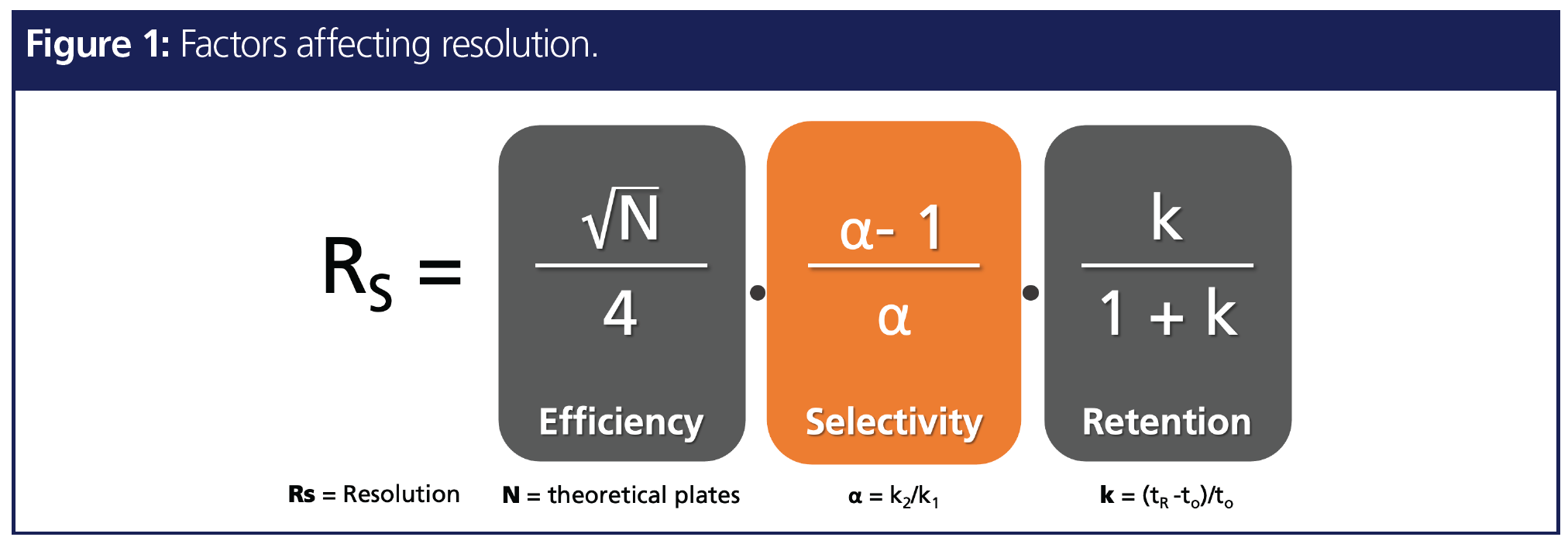
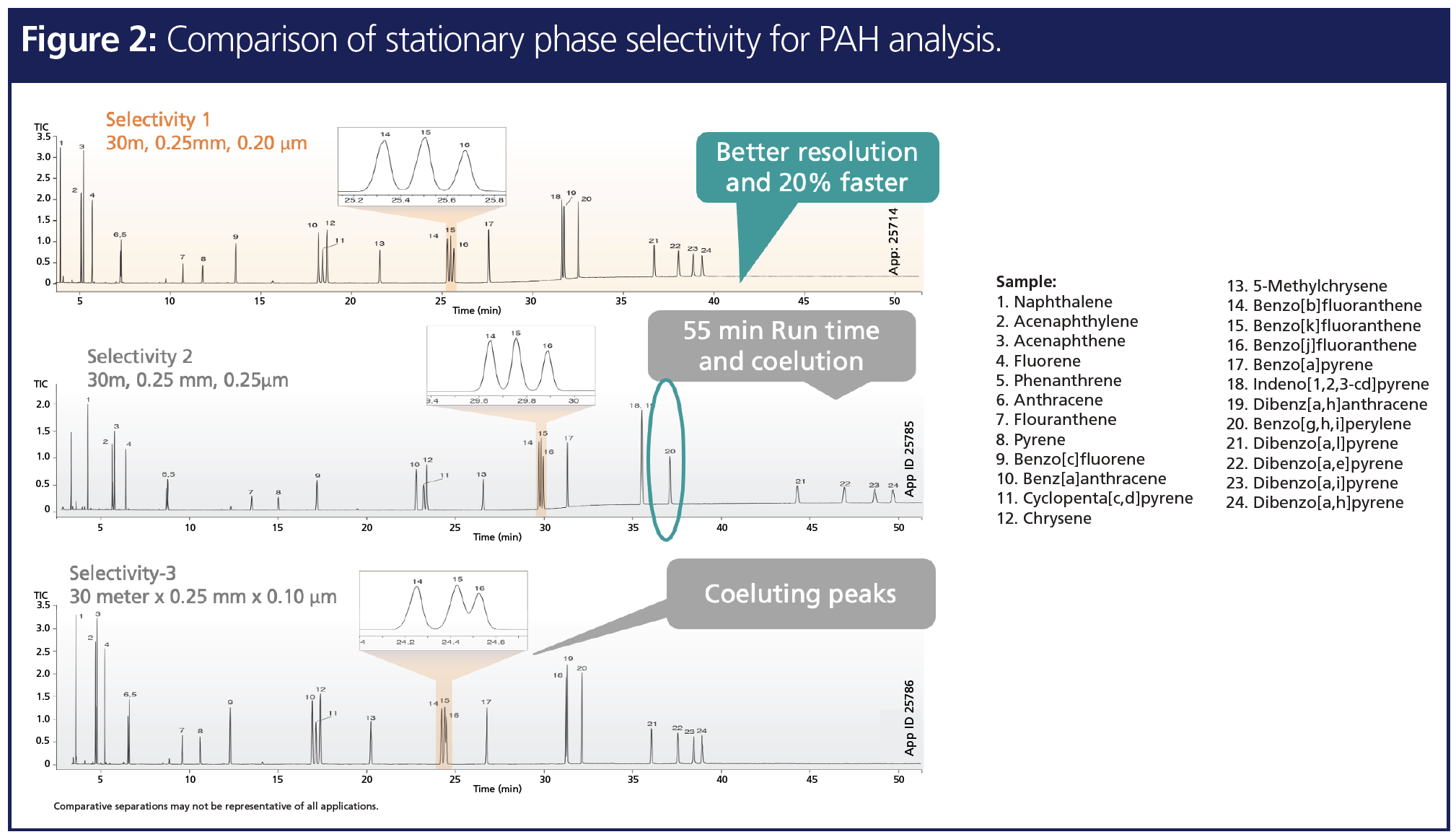
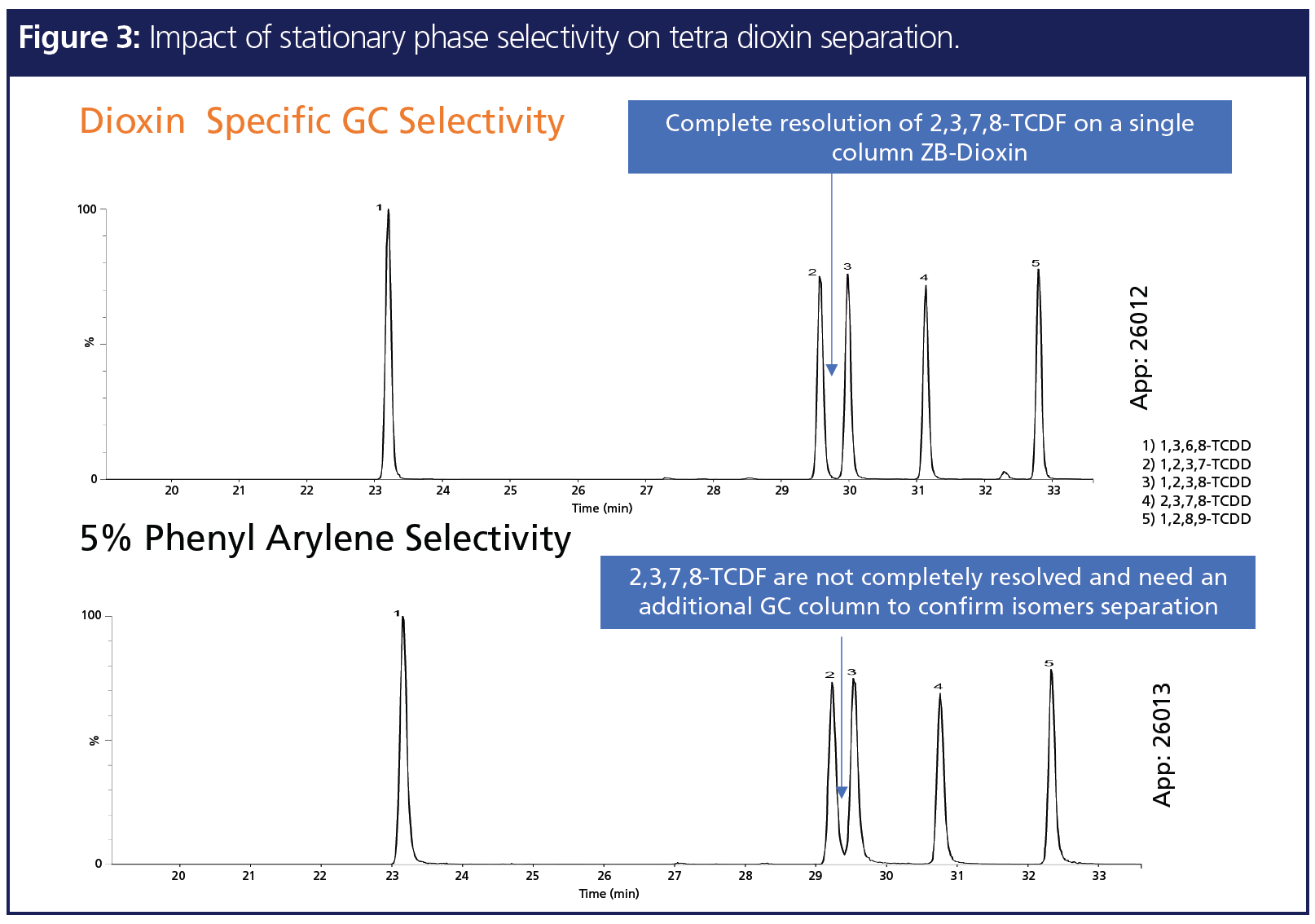
Figure 3 represents another example proving selectivity is most influential for resolution improvements for GC analysis. In dioxin analysis by GC–high-resolution mass spectrometry (HRMS), special attention is given to 2,3,7,8-TCDD separation from the rest of the isomers (4). Compared to a 5% phenyl arylene-based stationary phase, a dioxin-specific stationary phase with higher percentage phenyl provides better resolution. In fact, this enhancement in resolution of critical pairs would exceed the 25% valley separation method requirement from EPA‑1613 for TCDD isomers (5).
2. Group Selective
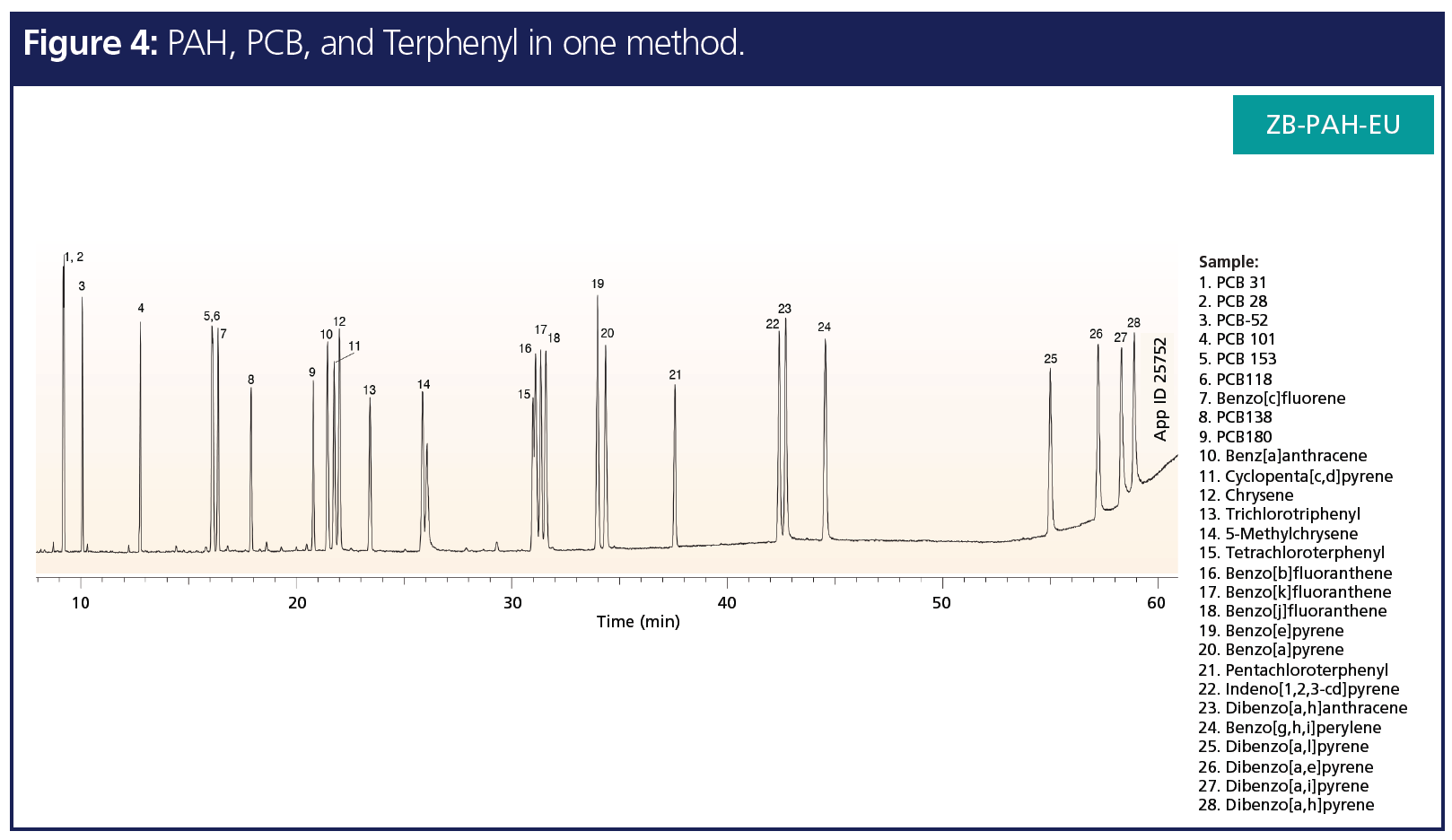
While analyte-specific resolution and selectivity are the most important, as a mass spectrometry user minimal change in columns between analysis is preferred to reduce downtime. Many times it is not efficient to run a single sample on several different columns with different selectivities to resolve different groups of analyte types. Being able to separate and analyze many different analyte classes on a single method/column allows for a reduction in downtime, as well as provide the ability to have a high‑throughput method for processing more samples in a given period. Figure 4 shows a representative chromatogram of a PAH-specific GC column with a highly selective stationary phase being able to recognize subtle differences in aromatic structures that resolves three different class of compounds, PAHs, PCBs, and terphenyls, in a single run method. Hence, choosing the correct, highly selective column can save resources and time for separation methods involving several different analyte classes.
3. Peak Shape Enhancement
Gas chromatography follows the “like resolves like” principle. This essentially means that polar analytes have better resolution and peak shape when a polar stationary phase is used and that same idea is also true of nonpolar analytes. Proper peak focusing and wetting of stationary phase prevent overload and asymmetric peaks. As an example, Figure 5 shows the analysis of nitrogen and phosphorous pesticides run on two columns with identical dimensions, but one is a low polar 5% phenyl arlene column and the other is a midpolar, multiresidue analysis column. Bromacil, a polar analyte represented by peak 7, had poor peak shape when analyzed on the 5% phenyl arlene column due to polarity mismatch. When analyzed on the midpolar multiresidue column, symmetric peak shapes were produced for polar analyte bromacil, in addition to getting good peak shape for all other analytes. Many times in POPs analysis analysts deal with an expansive list of analytes, including polar and nonpolar components. So, choosing a moderate polar column gives the flexibility to achieve symmetric peak shape for both nonpolar and high polar compounds. In addition, in POPs analysis, it is also important to utilize a deactivated fused silica column to help enhance peak shape. This conceals active spots in the column that may be otherwise present in the stationary phase. Premium deactivation results in reducing analyte breakdown, which is important for thermally labile POPs such as DDT, endrin, and mirex (6).

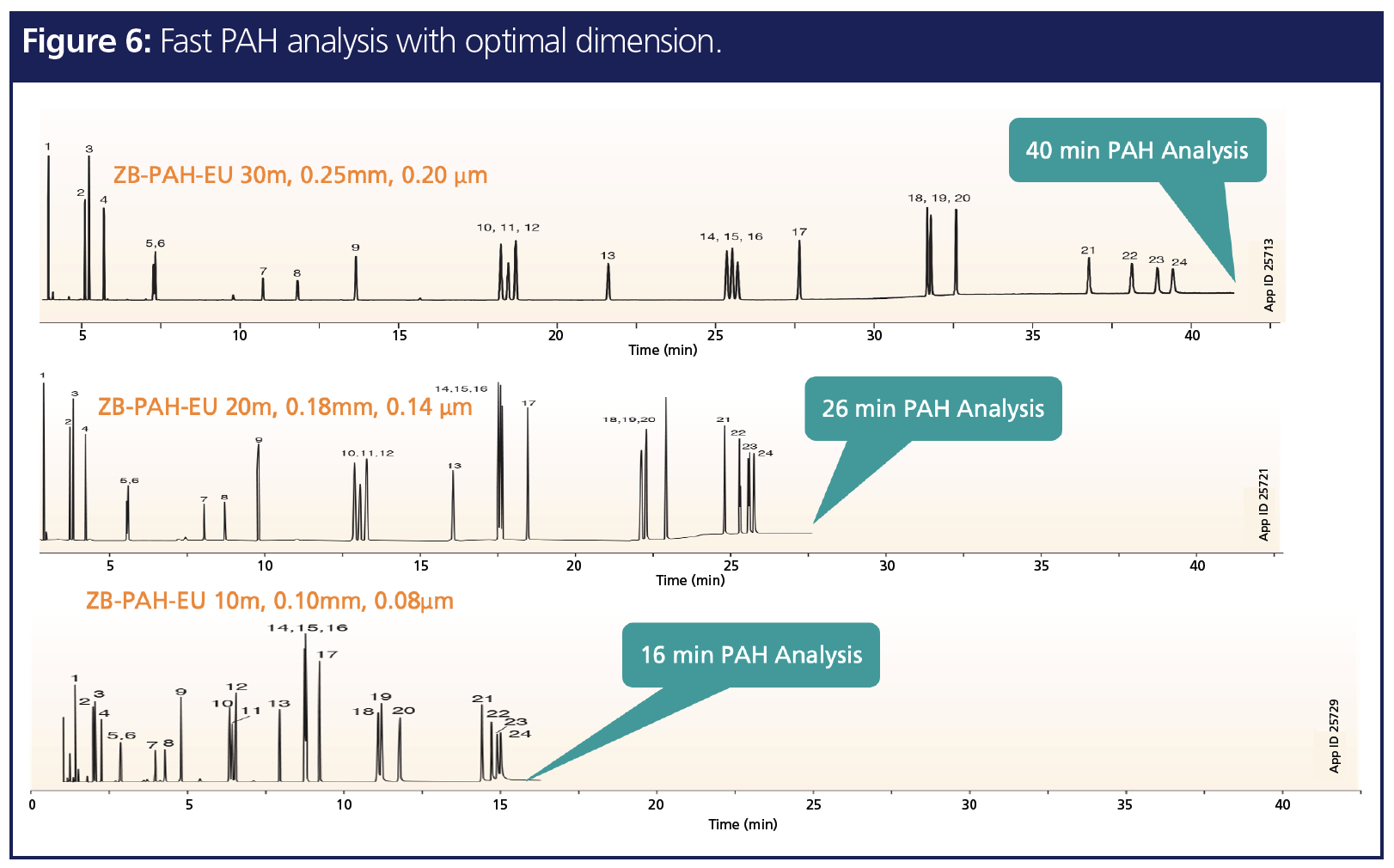
4. Fast Analysis and High Throughput
Many traditional methods for POPs analysis are very inefficient and time-consuming. There is a need for method development that will allow for an increase in the number of samples analyzed in a shorter amount of time. One way to address this need is by decreasing the inner diameter (i.d.) of a GC column. By decreasing the inner diameter, there is faster mass transfer, which results in higher efficiency. This means that a shorter length column can be used and a faster, yet still accurate, analysis will occur. Figure 6 shows a PAH analysis. On a 30 m × 0.25 mm, 0.2‑µm column, the run time for separation of all analytes was about 40 min. By decreasing the column inner diameter from 0.25 to 0.10 mm and length from 30 m to 10 mm, the run time was decreased, but resolution of peaks was maintained. This was achieved because the phase-volume ratio of the column stayed the same between the different sized columns. The caveat of decreasing column length and inner diameter is that the sample amount that can be loaded also decreases, which can affect resolution and separation of analytes. The user will have to strike a balance between column capacity (loadability) and run time to find the right methodology that will provide fast analysis, reliable results, and desired resolution.
5. Detector Selectivity and Sensitivity
Traditionally, POPs, like pesticides, were analyzed with a dual column system and an electron capture detector (ECD). For example, chlorinated pesticides would be run with a guard column Y splitter running to two separate GC columns and detectors. These two columns would have different selectivity so that all analytes would be resolved and detected with the ECD. Despite the column selectivity, there is still uncertainty with the possibility of matrix coelution and authentic quantitation. In addition, this solution doesn’t allow for low limits of detection. Modern methods use high‑end mass spectrometry with MS/MS capability or HRMS for authentic detection and low-level quantification of POPS including pesticides, PAHs, and PCBs. By utilizing spectral resolution in addition to chromatographic resolution, this helps to improve the quality of the analysis and to achieve separation of a wide range of POPs, using a single column for trace-level detection of analytes.
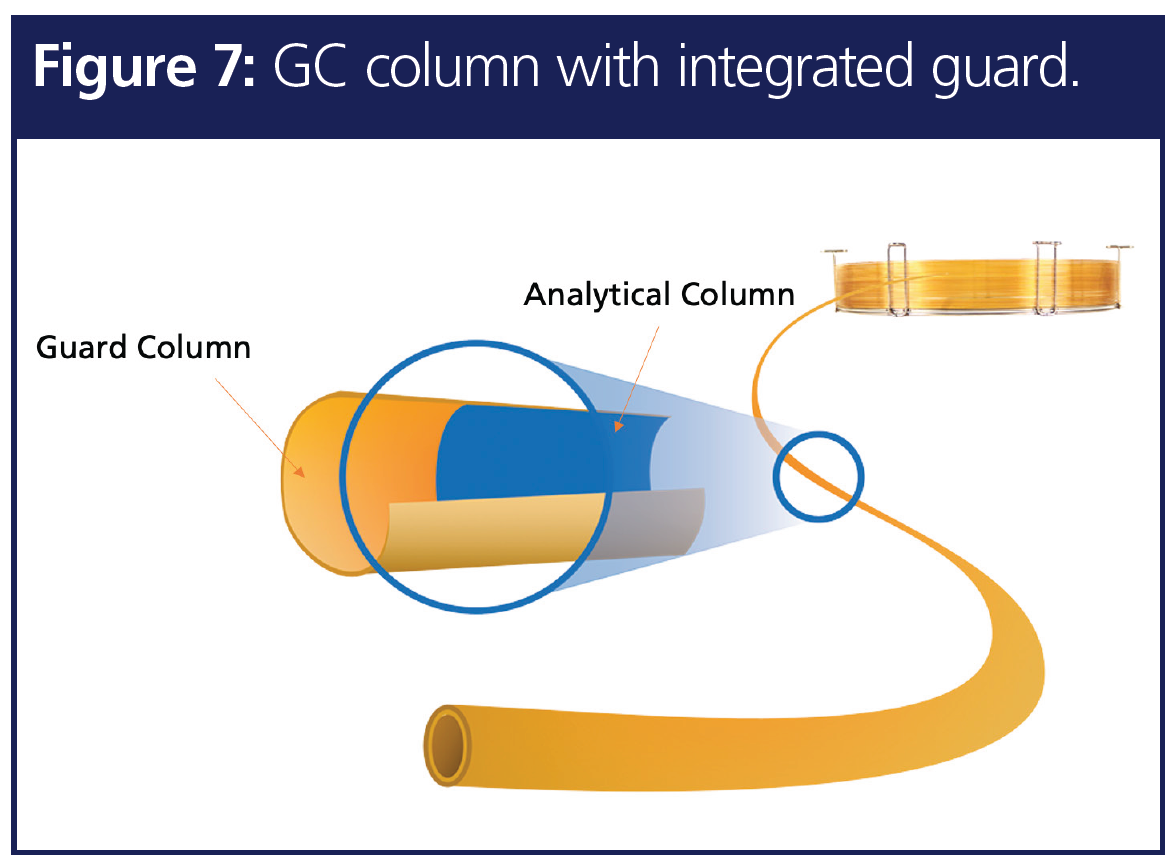
6. Peak Focusing Effect and Column Protection for Dioxin Testing
A common extraction mode for POPs testing involves liquid–liquid extraction (LLE) followed by preconcentration of the extract. This technique lacks selectivity for the analytes and can bring in matrix contaminants. While there is a requirement for low-level detection and sensitivity, it is common to see large volume injection in dioxin and other POPs analysis. Due to the extraction and preconcentration, semivolatile matrix can also enter and settle on to the GC column head. A guard column prevents nonvolatile contaminants from entering the analytical column, prevents excessive trimming of analytical column, extends GC column lifetime, improves peak focusing of early eluters by acting as a retention gap, and the seamless union of guard and analytical column provides a leak-free connection. Thus, a GC guard column helps improve the lifetime of the analytical column and improves separation in dioxin analysis. Since the guard portion is a deactivated fused silica tubing without stationary phase, it provides minimal retention time change compared to the analytical column (Figure 7).
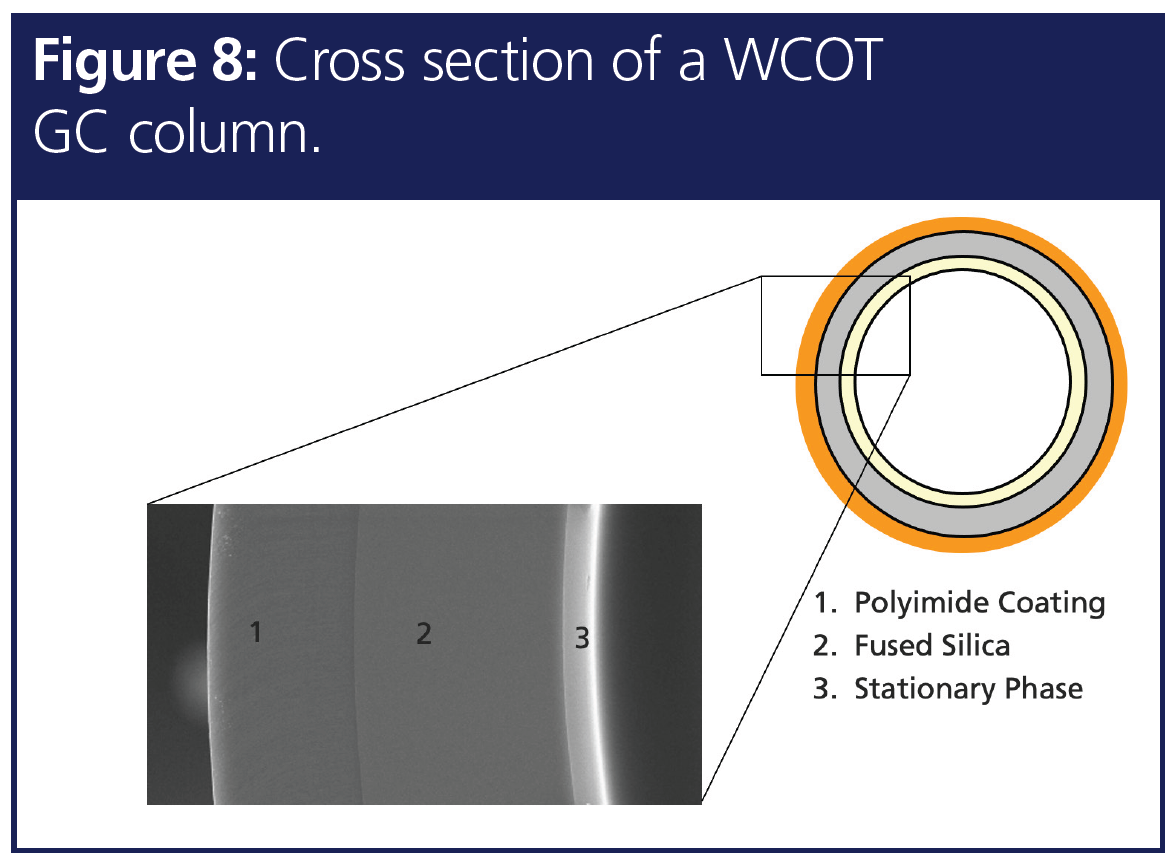
7. Mass Spec Compatibility
Modern POPs testing involves high resolution GC separation followed by MS, HRMS, or MS/MS. In GC mass spec methods, the transfer‑line is often kept at an elevated temperature constantly to avoid reverse condensation of vapours. It is therefore essential to have a GC column that is mass spec-compatible. Figure 8 represents the cross section of a GC column where the innermost layer stationary phase needs to have adequate cross-linkage and proper surface deactivation of the middle layer in order to get lower bleed. A method-specific phase is preferred in most applications and, in this case, a column that undergoes extensive cross-linkage of the stationary phase to provide low bleed and mass spec compatibility facilitates a seamless dioxin separation. In addition, MS compatibility ensures low baseline noise and eventually increases signal-to-noise ratio to improve sensitivity.
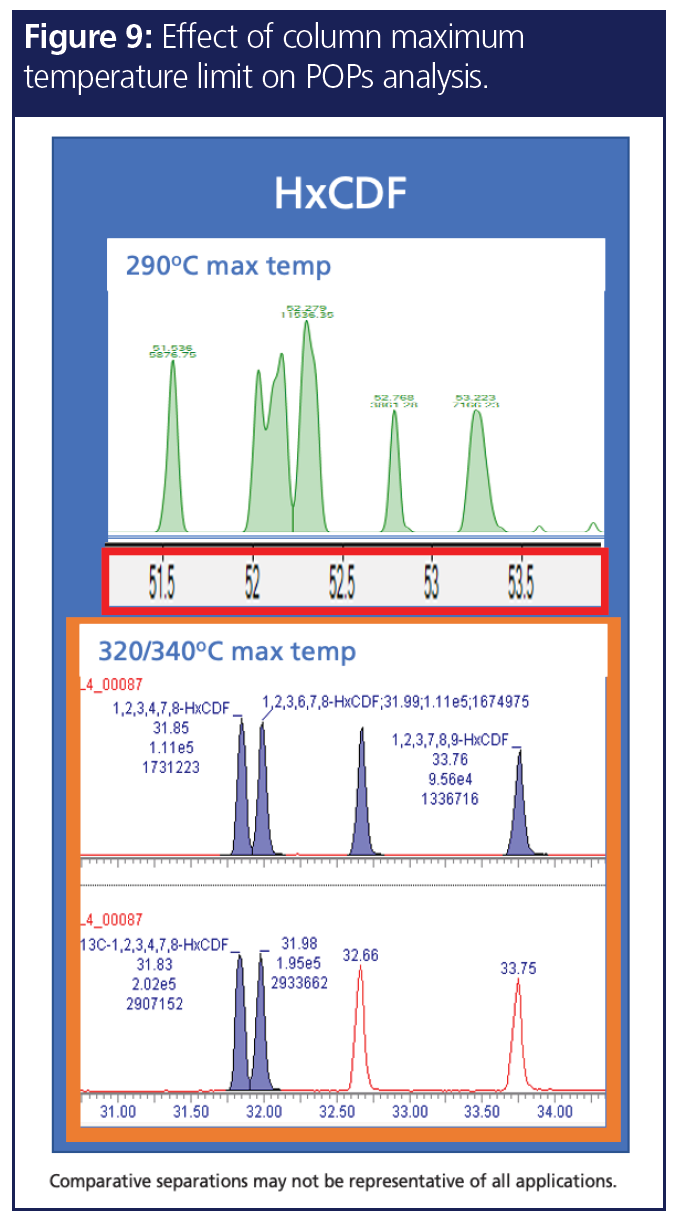
8. Temperature Stability
A GC column’s temperature stability decides the maximum temperature that can be set in a GC method. As shown in Figure 9, temperature stability can play a crucial role in POPs analysis. The top chromatogram shows dioxin analysis with high-boiling hexafurans eluting on a dioxin-specific column with 290 °C as the maximum temperature. Since the hexafurans are late eluters, they suffer from diffusion due to their slow elution at 290 °C. By using a dioxin-specific GC column that can withstand 320/340 °C, the peaks are sharper, the late eluters are well separated with better sensitivity, and the analytes elute faster thereby providing shorter analysis time. It is therefore equally important to choose the right selectivity and a column with high temperature limits to get better sensitivity, resolution, and a short run time.
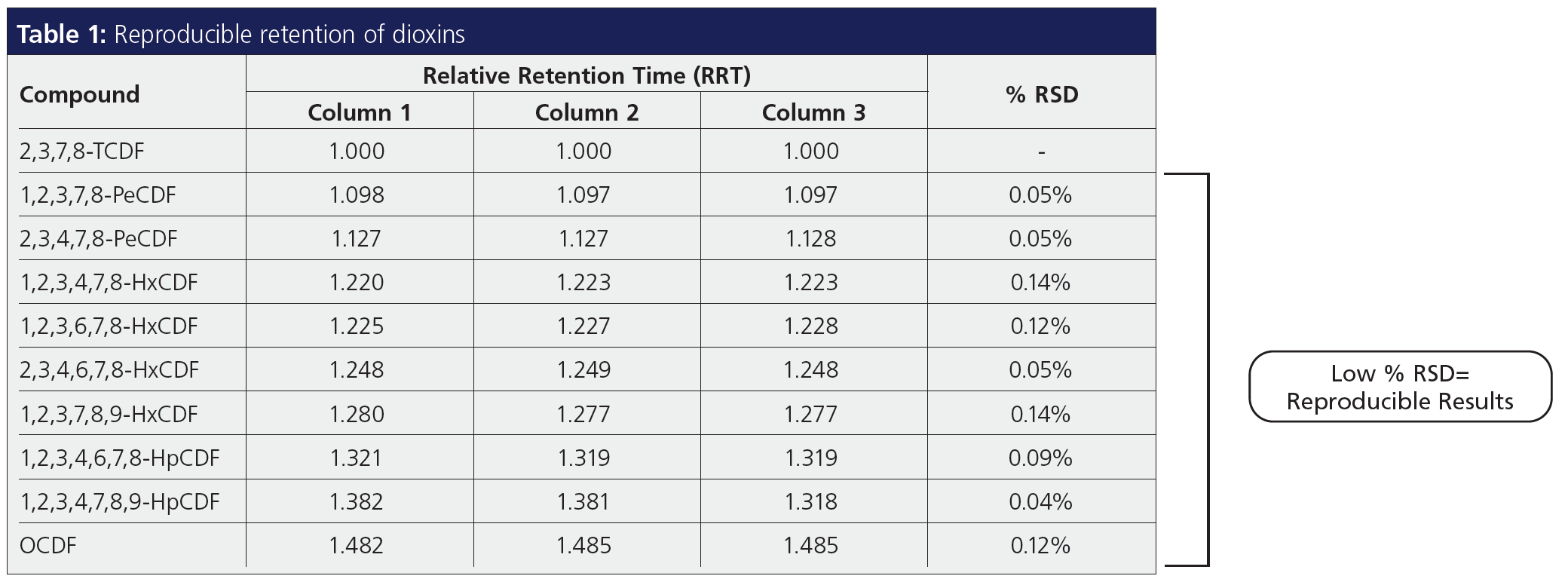
9. Reproducibility and Robust Analysis
Column-to-column reproducibility is extremely important for POPs analysis separation. EPA methods such as 1633 (4) have specific resolution requirements for critical pairs. Represented in Table 1 is the reproducibility of dioxin isomers on a dioxin‑specific column. The low percentage relative standard deviation (RSD) for relative retention time signifies minimal variability from column to column. As noticed, it is very important to consider a stationary phase that is specially tailored for dioxin testing. A generic phase such as a 5% phenyl phase may be optimized for polarity; they are tested with generic probe analytes, so there is no guarantee that the critical resolution would be met every time. On the other hand, method-specific GC columns for PAH, PCB, and pesticide analysis are tailored to meet and exceed method requirements.
10. Reliable Quality
Users rely on quality columns to produce the best results from their analysis. In most cases, shortcuts may be adopted by batch testing these columns. This gives an assumption that all columns in that batch are reliable if the columns in the test round pass inspection. However, for critical analysis like POPs, it is important to consider a column that is ready to use right out of the box. Therefore, choose a reliable GC column that provides assurance on testing individual columns with subjective evidence on the certificate of analysis.
Conclusion
POPs GC analysis is critical to monitoring the quality of environmental and food samples across the world. With evolving standards and a focus on POPs, it has become a vital part of regulation testing. When selecting a GC column or method requirements, it is important to consider the various aspects discussed above and weigh up the options to produce the most accurate results. The parameters for the analyses outlined display an optimal selection for a robust and reproducible analysis by GC, all requiring a high‑quality GC column to accomplish it.
Reference
- https://www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response
- https://www.epa.gov/sites/default/files/2015-10/documents/method_610_1984.pdf
- https://www.phenomenex.com/Documents/2020/09/24/14/09/Zebron-ZB-PAH-EU-and-ZB-PAH-CT-GC-columns-Guide
- https://nepis.epa.gov/Exe/ZyNET.exe/9100E9BU.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1986+Thru+1990&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C86thru90%5CTxt%5C00000021%5C9100E9BU.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL
- https://www.phenomenex.com/Documents/2022/05/20/18/57/Zebron-ZB-Dioxin-Application-Guide
- https://www.phenomenex.com/products/zebron-gc-columns/zebron-zb-multiresidue-1
Ramkumar Dhandapani has been in the chromatography industry for over 20 years and has hands-on and troubleshooting experience in chromatography. He has earned a master’s degree and doctoral degree in analytical chemistry from Seton Hall University, New Jersey, USA, with specialization in microextractions, multidimensional gas chromatography, and tandem mass spec techniques. He has developed and validated several regulatory compliant methods in the pharmaceutical, food, and environmental industries, as well as incorporated method improvement and troubleshooting across a range of GC techniques. He joined Phenomenex in August 2014 and serves as Global Product Manager-Gas Chromatography at Phenomenex, USA. In addition to managing the GC product line, he presents on innovations in GC at various chromatography conferences.
Bryan Tackett earned a bachelor’s degree in biochemistry and genetics from Texas A&M University (Texas, USA) and a doctoral degree in translational biology and molecular medicine from Baylor College of Medicine (Texas, USA). He joined Phenomenex in September of 2020 and he serves as Technical Marketing Writer at Phenomenex, USA.
E-mail: ramkumard@phenomenex.com
Website: www.phenomenex.com/Techniques/GC-Application-Specific

Distinguishing Alcohol- from Non-Alcohol-Associated Liver Cirrhosis with LC-MS
May 7th 2025A pilot study investigating whether nicotinamide adenine dinucleotide kinase (NADK) expression is selectively diminished in alcohol-associated liver cirrhosis (AC), as well as evaluating its potential as a biomarker for this condition, measured AC and non-AC (NAC). Nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+) levels in human liver samples were measured using liquid chromatography-mass spectrometry (LC-MS).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














