Tips & Tricks: Column Order in GPC/SEC
To provide a large molar mass separation range in gel permeation chromatography/size-exclusion chromatography (GPC/SEC), columns of different pore sizes are frequently combined. This raises the question of whether column order influences resolution. This instalment of Tips & Tricks will discuss the effect of column order on separation efficiency.
Gel permeation chromatography/size-exclusion chromatography (GPC/SEC) separates macromolecules based on their hydrodynamic volume. The mechanism of GPC/SEC involves diffusion-driven distribution of the macromolecules between the flowing eluent and the stagnant eluent in the pores of the column’s packing material. Macromolecules smaller than the pore size can enter the pores, while for large molecules the pores are too small to allow for penetration. Consequently, large molecules elute sooner than smaller ones.
If the sizes of the macromolecules exceed the size of the pores, all molecules elute at the same elution volume. The exclusion limit is reached. Similarly, for molecules much smaller than the pores, separation efficiency is low. The best resolution therefore is achieved if the macromolecules are of a similar size to the pores.
As a rule of thumb, a column filled with particles of a uniform pore size provides a separation range of approximately two orders in molar mass (1,2). If the molar mass distribution of the sample covers a larger molar mass range, the packing of the columns applied must provide a range of different pore sizes. This can be realized by using either columns filled with mixtures of particles with different pore sizes (mixed bed or linear columns), or by coupling several columns—each having a different average pore size (column combination, column bank). The advantages and disadvantages of the two different approaches have been discussed previously (3).
When combining columns of different pore sizes certain questions arise, such as which column should be installed closest to the injector or the closest to the detector?
The Effect of Variation of Column Order on Polystyrene Calibration Curve and Elution Volume of Polystyrene Standards
To investigate this question, a set of styrene‑divinylbenzene-based columns with pores sizes of 103, 105, and 106 Å was applied. To avoid changes of dead volume when changing the column order, an eight-port two-position valve was installed, allowing the positions of the first and last column to be exchanged without disassembling and reassembling the column bank. However, flow direction for all columns was maintained. Mixtures of polystyrene standards were injected in THF at a flow rate of 1 mL/min. Detection was performed using UV at 254 nm. An injection volume of 20 µL and a total concentration of 3.5 g/L was chosen.
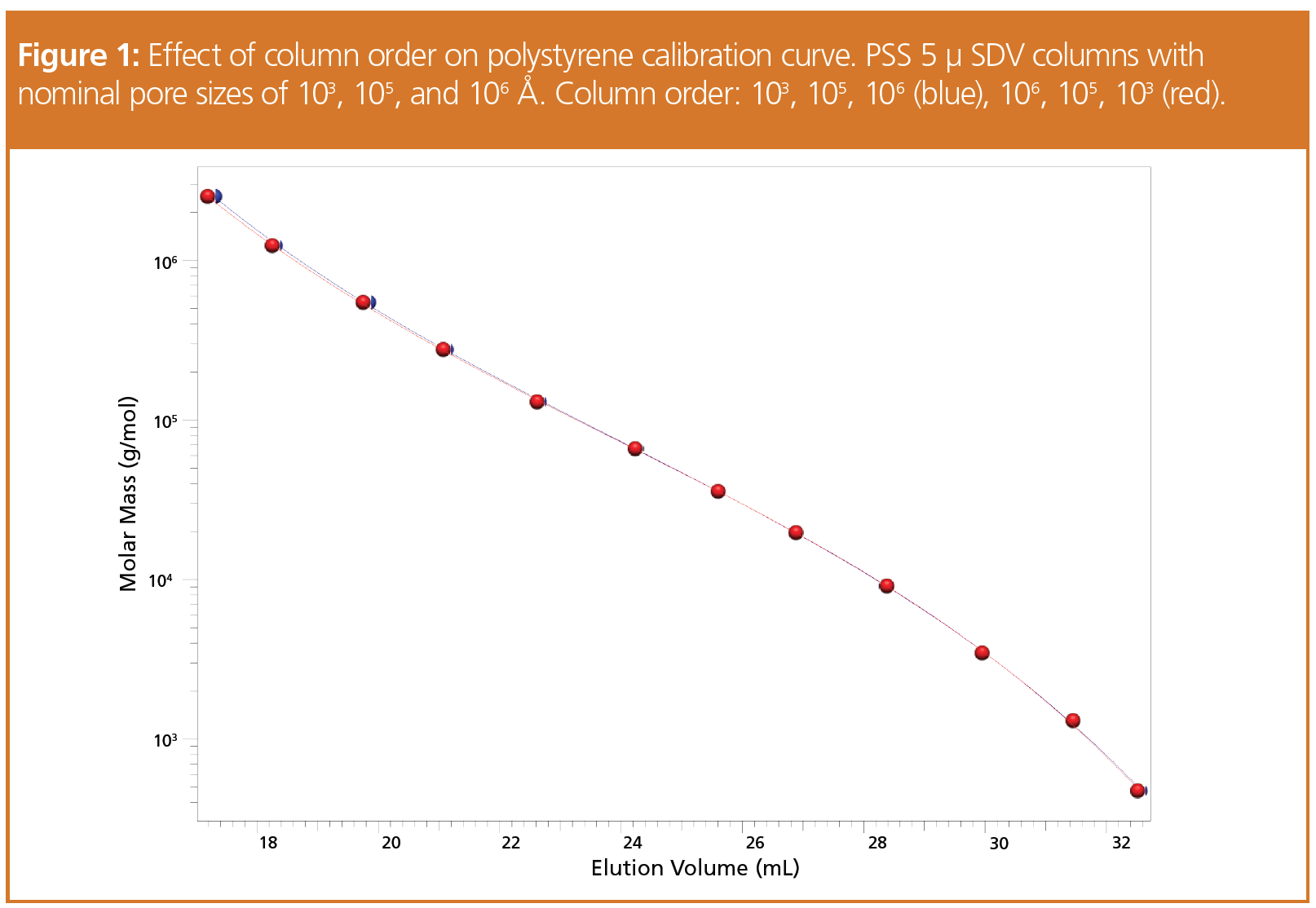
Figure 1 shows the calibration curves for polystyrene standards obtained for two different arrangements of the same columns. Obviously, the calibration curves superimpose nicely. Only for the very high molar masses does a weak deviation appear to be visible. Thus, the column order had only a weak impact on the calibration curve, if at all.
Effect of Variation of Column Order on Resolution of Polystyrene Standards
A closer look was given to the individual chromatograms. Figure 2 and Figure 3 show the chromatograms for two sets of polystyrene standards obtained using different column orders. In both cases very similar separations were achieved. Only the highest molar mass standards revealed a weak shift to lower elution volumes when the columns were installed in order of decreasing pore size. The effect decreased with diminishing molar mass.
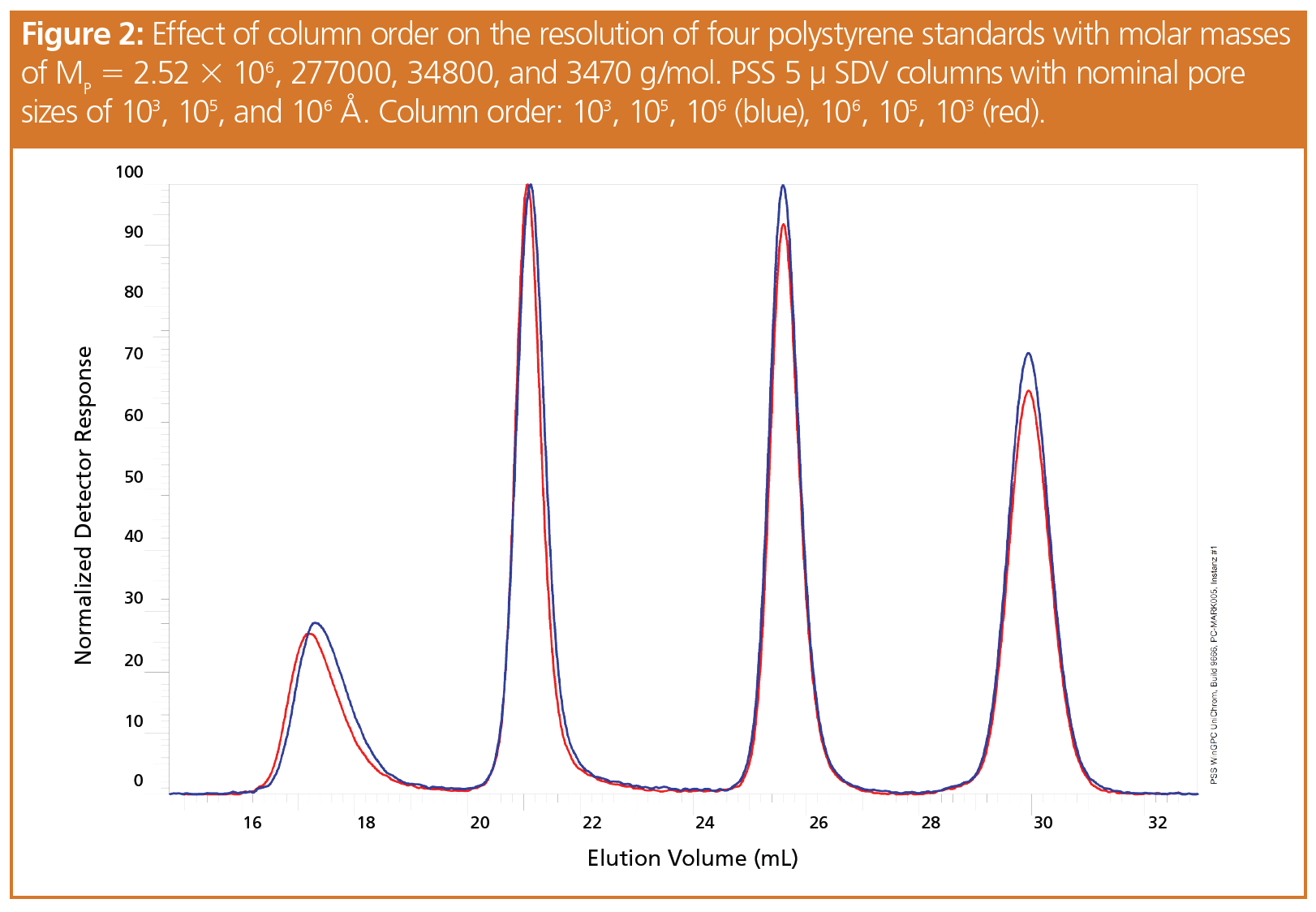
As the elution volume difference between the high and low molar mass standards increased when installing the large pore size columns first, the resolution was enhanced compared to the inverse column order.
The fact that the high molar mass samples eluted slightly earlier than those of lower molar mass when the largest pore size column was placed first might be attributed to column overloading, resulting in a too high local concentration.
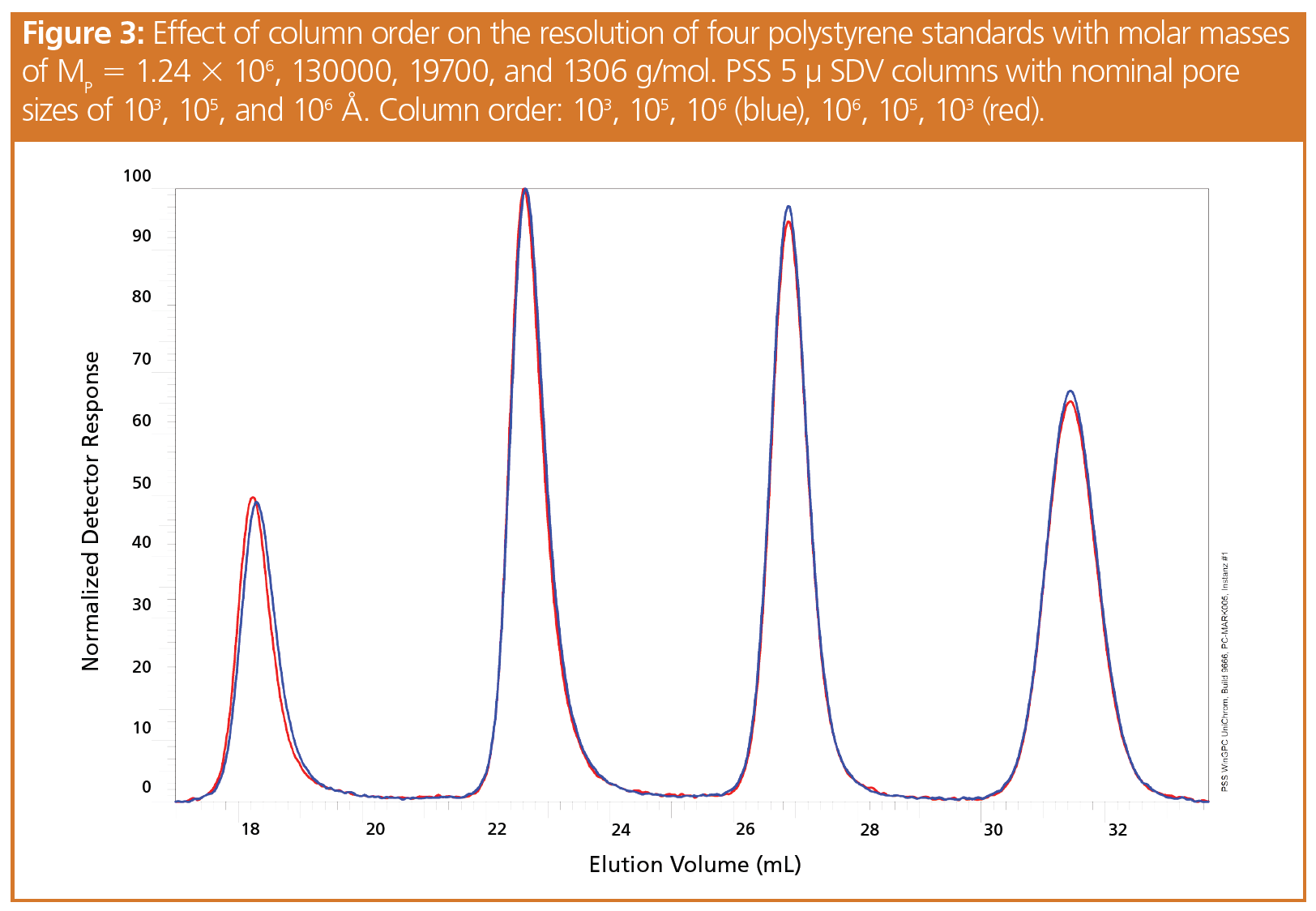
When the first column was of a small pore size, molecules exceeding the first column’s exclusion limit were not separated from each other and all eluted together from the first column. They were therefore introduced onto the second column at a rather high concentration.
In contrast, if the large pore size column was placed first, the high molar mass species were rapidly separated from each other and their concentration was quickly reduced. Thus, the high molar mass components entered the second and all following columns at relatively low concentration. Because the large pores of the first column were less effective for the separation of the low molar mass species, those entered the second column at comparably high concentrations.
However, the injection of high molar mass macromolecules at high polymer concentrations is known to result in a slightly retarded elution (4), while the retention volume of low molar mass macromolecules is less affected by concentration. Therefore, it is understandable that a change in column order affects the elution of the high molar masses more strongly and has a negligible effect on the lower molar mass molecules.
However, it should be stressed that the effect of column order on retention volume is low in general, and only becomes more pronounced when approaching the column combinations’ exclusion limit.
The above observations might suggest installation of the large pore size column close to the injector and the small pore size column at the end of the column bank. However, larger pore size columns are usually less pressure‑resistant. For this reason, it is recommended to install the columns in order of increasing pore size. Only when analyzing very high molar masses should column order be changed. As very large macromolecules are prone to flow‑induced chain elongation and degradation, such analysis should be run at lower flow rates anyway (4), reducing the risk associated with high back pressure on column stability.
Summary
- Varying the column order in GPC/SEC influences the shape of the calibration curves only marginally at otherwise
identical conditions. - For high molar mass molecules, the resolution is slightly improved if large pore size columns are followed by columns of smaller pore size.
- However, columns should preferably be installed in order of increasing pore sizes because of stability arguments.
- Only when analyzing very high molar mass samples should the column order be reversed. As a further precaution for samples of high molar mass, the analysis should be performed at lower flow rates and low concentrations.
References
- W. Radke, Macromol. Theory Simul. 10(7),
668–675 (2001). - E.F. Casassa and Y. Tagami, Macromolecules 2, 14 (1969).
- D. Held and W. Radke, The Column 17(4), 26–30 (2021).
- D. Held, The Column 10(10), 12–15 (2014).
Wolfgang Radke studied polymer chemistry in Mainz (Germany) and Amherst (Massachusetts, USA) and is head of the PSS application development department. He is also responsible for instrument evaluation and for customized trainings.
E-mail: WRadke@pss-polymer.com
Website: www.pss-polymer.com

Influence of Concentration in Conventional GPC/SEC and Advanced Detection GPC/SEC
March 21st 2025Sample concentration is a parameter that can influence the quality of gel permeation chromatography/size-exclusion chromatography (GPC/SEC) separations and the obtained results. Understanding this influence can help to support the development of reliable GPC/SEC methods.











