Tips & Tricks GPC/SEC: Calibration Using Broad Standards
Calibration using broad standards in GPC/SEC is discussed.
Volume 11 Issue 2
Pages 10-15
For polymeric materials where no narrowly distributed molar mass standards are available, reliable and true molar masses can be obtained using well characterized samples of broad molar mass distribution. This approach does not require additional instrumentation and offers an alternative to using molar mass sensitive detectors such as on-line light scattering or on-line viscometry detectors.
Gel permeation chromatography/size-exclusion chromatography (GPC/SEC) is an easy and robust method to determine molar masses and molar mass distributions (MMD) of macromolecules. The separation is based on the size of macromolecules in solution - the larger the macromolecule, the earlier it elutes from the column. The primary information generated by GPC/SEC is the chromatogram showing the detector signal as a function of elution volume. To derive the molar mass distribution, the chromatogram has to be converted using a calibration curve that relates elution volume to the molar mass of the eluting polymer. Calibration curves are usually established using narrowly distributed polymer standards; however, chemically different polymers of the same molar mass often exhibit very different sizes in solution. Correct molar masses are therefore only obtained if the chemical structure of the calibrant matches the structure of the analyte.

Photo Credit: CGinspiration/Getty Images
Unfortunately, for a large number of important polymers (for example, polyamides, polyesters, or polyolefins), narrow standards are not commercially available and alternative methods are required. The use of a molar mass sensitive detector is one option but it requires additional expensive instrumentation, more precise and accurate sample preparation, more time, and a greater level of knowledge about data evaluation.
These limitations are especially crucial for quality control laboratories because fast, robust, and easy-to use calibration methods are required. A suitable, cost-effective solution is the use of broadly distributed standards.
Calibration Using Broad Samples - How Does It Work?
For a narrowly distributed polymer, the maximum peak of the chromatogram can be easily identified and assigned. In addition, we can assume Mw ≈ Mn ≈ Mp. This is not the case for a broadly distributed sample. Here, the maximum can be difficult to precisely identify because it can depend on the resolution of the separation columns. It is therefore not recommended to establish the calibration curve by simply plotting the molar masses versus the elution volume at peak maximum.
The generally accepted concept of universal calibration1 in GPC/SEC results in the following relationship for the calibrations curves of the calibrant and the analyte:
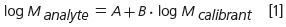
This is based on the assumption that both calibrant and analyte elute without interacting with the stationary phase. The parameter A shifts the calibration curve vertically relative to the calibration curve, while a change in B varies the slope of the calibration curve (Figure 1). The parameter A and B are related to the Mark–Houwink parameters of the analyte and calibrant in the respective solvent.2–5 Because of the effect of parameters A and B on the calibration curve, the MMD and consequently the molar mass averages calculated for a given chromatogram change as a function of the parameters as well.
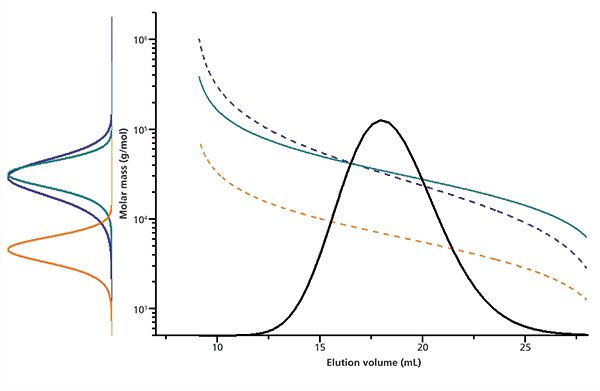
Figure 1: The effect of parameters A and B on the calibration curve and subsequently MMD. Solid turquoise: Original (base) calibration curve and MMD (left) derived therefrom. Blue: Calibration curve and MMD derived from turquoise curve by changing parameter B (slope). Orange: Calibration curve and MWD derived from blue curve by changing parameter A (vertical shift).
This is illustrated in Table 1 where two chromatograms of broadly distributed poly(lactic acid) (PLA) samples were evaluated using a polystyrene calibration curve (base calibration) and different sets of A and B. Application of the polystyrene calibration curve overestimates the true molar masses by approximately a factor of 3. Changing the parameters brings the molar mass closer to the true value; however, both parameters need to be changed to get reliable results for different samples. For the optimized parameter set A and B, given in the last line, a good agreement with the reference values from light scattering is observed.
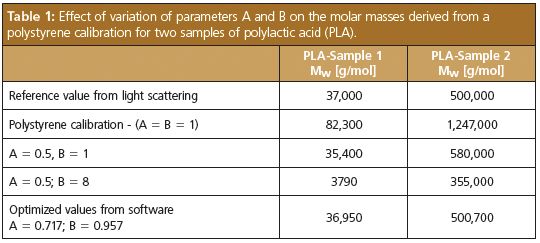
Table 1: Effect of variation of parameters A and B on the molar masses derived from a polystyrene calibration for two samples of polylactic acid (PLA).
Table 1 also explains how the parameters A and B can be determined from the chromatograms and known average molar masses of broadly distributed standards. To obtain the A and B, they are systematically varied, until the best agreement between the known molar masses and the ones calculated from the respective chromatograms, the base calibration, and the parameters is obtained.
Fortunately this variation can be automated with GPC/SEC software packages. With the obtained parameters, a suitable calibration curve for unknown samples of the same structure as the analyte can be easily derived from the base calibration.
In contrast to other approaches for broad standards calibration, the above approach benefits from the fact that the base calibration reveals the pore size distribution of the column packing and therefore the true shape of the calibration curve. In particular the method preserves the non-linear shape, the exclusion volume, and the separation limit of the base calibration curve. An additional advantage is that more than one sample can be used to create the calibration curve. This increases accuracy and allows a wider molar mass range to be covered.
How to Establish a Calibration Using Broad Standards
Establishing a calibration curve by the above procedure requires:
- A base curve established using narrowly distributed standards of arbitrary chemical structure, such as polystyrene for many organic solvents or pullulan of poly(ethylene oxide)/poly(ethylene glycol) (PEO/PEG) for aqueous applications.
- At least one broadly distributed sample with known Mw and Mn values of the same chemical structure as the analytes to be characterized. If two or more broadly distributed samples are used one molar mass average (such as Mw or Mn) per sample is sufficient.
The procedure then is straightforward:
- Run the narrowly and the broadly distributed standards on your GPC/SEC system.
- Establish the base calibration using the molar masses (Mp) and peak maxima of the narrowly distributed calibrants.6
- Use a software tool to shift the calibration curve and adjust the slope so that the calibration provides correct results for the broadly distributed samples. This means that the software internally determines the optimized parameters A and B using the chromatograms of the broad standards and applying the known molar mass averages.
- Save this calibration curve so that it can be used to determine the complete molar mass distribution and the true molar mass averages of samples with unknown molar masses.
Figure 2 shows the chromatograms of the broadly distributed standards as well as what is required to use the broad standards calibration concept. To increase the accuracy of the calibration, more than one broadly distributed standard should be used. If a wide molar mass range needs to be covered the use of several samples is recommended.
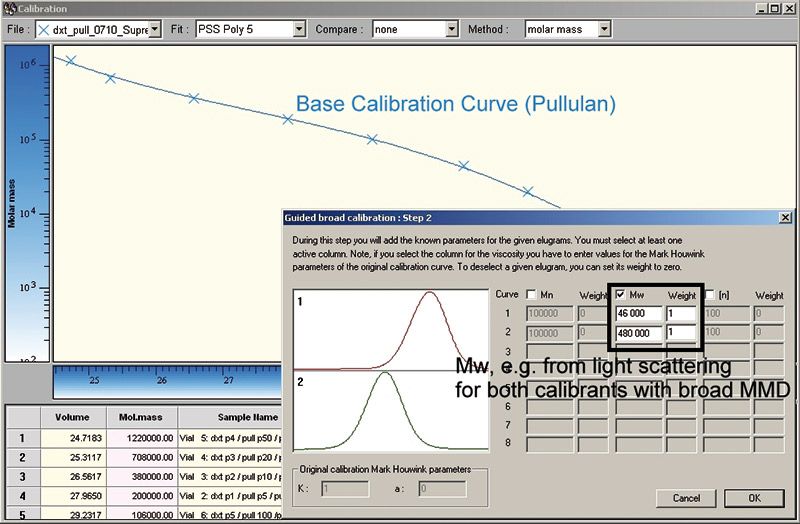
Figure 2: Example for a base calibration for two samples of polylactic acid (PLA) with known Mw from light scattering. To increase the accuracy and the molar mass range, up to eight different samples can be used.
Figure 3 shows a comparison of the base calibration (blue) and the resulting calibration curve after determination of A and B (red). This calibration curve is then used for the evaluation of unknown samples and it provides true molar masses for all samples of this type.
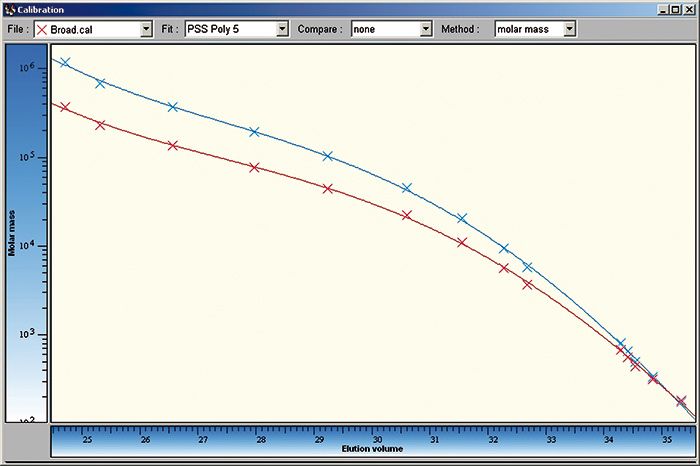
Figure 3: The blue calibration curve is the pullulan base calibration curve. If this curve is used for evaluation molar masses are overestimated. The red curve is the resulting calibration curve calculated using the optimized A and B values from the samples with the broad molar mass distribution.
How to Get Broad Standards
A limited number of broad standards for a variety of chemical structures is available from suppliers. If no broad standards are commercially available, sample testing laboratories or the R&D department can determine true molar mass using conventional batch measurements for example, by light scattering, nuclear magnetic resonance (NMR), or osmometry. On-line determination using multidetection GPC/SEC can also solve this problem.
Summary
- Calibration using broad standards is a fast and easy-to-use method for quality control laboratories.
- Standards with a broad molar mass distribution can be easily obtained from contract analysis laboratories or the R&D department using batch methods. This allows the measurement of precise and accurate molar masses worldwide.
References
1. Z. Grubisic, P. Rempp, and H. Benoît, Journal of Polymer Science, Part B: Polymer Letters5(9), 753–759 (1967).
2. S. Mori, Anal. Chem. 53, 1813–1818 (1981).
3. H.K. Mahabadi and K.F. O´Driscoll, J. Appl. Polym. Sci. 21, 1283–1287 (1977).
4. A.R. Weiss and E. Cohn-Ginsberg, Journal of Polymer Science Part B: Polymer Letters7(5), 379–381 (1969).
5. W. Radke, in Macromolecular Engineering, K.G. Matyjaszewski and L. Leibler, Eds. (Wiley-VCH, Weinheim, Germany vol. 3), pp. 1881–1936.
6. D. Held, The Column4(6), 18–21 (2008).
Daniela Held studied polymer chemistry in Mainz, Germany, and works in the PSS solutions department. She is also responsible for PSS webinars, education programmes, and customer training.
Wolfgang Radke studied polymer chemistry in Mainz, Germany, and in Amherst, Massachusetts, USA. He is now head of the PSS application development department. He is also responsible for the PSS customer training programme and for customized trainings.
E-mail: Dheld@pss-polymer.com
Website: www.pss-polymer.com
This article is from The Column. The full issue can be found here>>

What Goes in a CDS IT Service Level Agreement?
Published: April 7th 2025 | Updated: April 7th 2025Protecting your network chromatography data system (CDS) data is critical and a service level agreement (SLA) with your IT provider is vital. What should be included? Are SLAs for in-house IT and SaaS (software as a service) similar?
Advanced LC–MS Analysis for PFAS Analysis in Eggs
October 11th 2024The European Commission's regulation on maximum levels for certain contaminants in food highlights the need for precise and reliable methods to quantify per- and polyfluoroalkyl substances (PFAS) in various food matrices. This article discusses development and validation of a robust method for analyzing 21 PFAS compounds in chicken eggs using solid-phase extraction (SPE) and liquid chromatography–mass spectrometry (LC–MS).






