Tips and Tricks for New Gas Chromatographers
LCGC North America
Three main points summarize the best ways to be successful in GC:capillary GC is clean GC; practice, practice, practice; and be a student of GC.
Three main points summarize the best ways to be successful in gas chromatography (GC) Capillary GC is clean GC; practice, practice, practice; and be a student of GC.
I am honored to be a guest author for the "GC Connections" column that I have read regularly for more than 20 years. This is my first experience writing a magazine column, and as I tried to figure out how to do it I thought it appropriate that the subject be on tips and tricks. I knew some of these when I was first learning gas chromatography (GC), and others I wish I knew then. New chromatographers, their managers and trainers, and those purchasing and installing a new system should benefit from these tips.
Three main points summarize the best ways to be successful in GC: Capillary GC is clean GC; practice, practice, practice; and be a student of GC.
Your GC work will be successful if you take the time to learn GC as a craft as well as a science. Work carefully to ensure your system is clean, and eliminate contamination. Practice the special lab skills for GC. As a first step, if you are reading someone else's copy of LCGC North America, sign up for your own subscription. I still read it regularly, and I like the print version. I scan it immediately each month when it arrives. As I was preparing this article, I noted five additional tips and tricks from ChromAcademy's online platform, discussed in the October 2017 issue of LCGC North America (1).
"Capillary GC is Clean GC"
One of the first lessons I learned about capillary GC is that cleanliness is critical. The quote "Capillary GC is clean GC" is from the first short course I took in the late 1980s and it has stuck with me since then. In capillary GC, the mass of an analyte is often very small (nanograms) and the mass of the stationary phase is small (milligrams at most), so any contamination can be a problem. As seen in most troubleshooting guides, contamination, especially at the inlet or prior, is the biggest cause of problems in capillary GC. Here are some tips and tricks that can help you reduce contamination before it affects performance and some tips for addressing contamination if performance is already suffering.
Check Your Inlet
The most common location for contamination is the inlet. Inlet contamination often causes a degradation in peak shape (tailing) that slowly gets worse as the contamination builds up. Three common locations for contamination that can be removed easily are just below the septum, in the glass sleeve, and at the bottom of the inlet. Most polymeric septa are good for 30–50 injections, so a regular schedule of septum changes is often required. The next time you change a septum, look at the bottom. If you see residue at the needle hole, it is contamination that can sometimes show up as ghost peaks. Glass sleeves should also be inspected and changed if they become dirty. Often, this contamination is seen as residue or film on the inside near where the sample is ejected from the needle, or as residue in any glass wool or packing material.
Finally, when changing the glass sleeve, it is a good idea to use a flashlight to inspect the bottom of the inlet. Small pieces of septum or broken column can collect there and cause contamination. If these are present or the bottom looks soiled, clean the area according to the instructions for your instrument. If cleaning the inlet does not eliminate the peak tailing or poor performance, then trimming the column may be necessary. If neither step eliminates the problem, then the column may need to be replaced.
Keep Your Detector Clean
Detectors are separately heated from the inlet and oven and are typically maintained at a high temperature to keep them clean. The idea is simple: Keep the column effluent in the vapor phase as it passes through the detector so analytes will not condense on and foul the detector surfaces. The most common indications that the detector needs to be serviced are an increase in noise or noise spikes, or a drop in sensitivity. These problems can be easily monitored by performing daily qualifications, which can be as simple as checking the baseline to see if it looks the same as it did yesterday. If changes are noted, such as higher baseline, more noise, or spikes, something has changed that may require maintenance.
For GC–mass spectrometry (GC–MS), monitoring the performance of a standard injected over time can identify the need for source cleaning. Steady loss of sensitivity or mass resolution usually indicates a contaminated source. Cleaning and maintaining most detectors is generally simple, but, as with most techniques in GC, it requires good lab skills and practice. If you determine that the detector needs to be cleaned or serviced, check the specific manual and instructions for your instrument or detector.
Use High-Purity Gases
Your GC uses separate gas supplies for the carrier gas and detectors, depending on what type is in use. The carrier gas (typically helium or hydrogen for capillary GC) should be high purity, and especially should be oxygen- and water-free. Oxygen and water, over time, can degrade the stationary phase or contaminate the detector. For detector gases, high purity is essential or the detector will become noisy, limiting sensitivity. Flame ionization detection (FID) requires hydrogen and air as fuel and oxidant for the flame. Some detection methods, such as electron-capture detection (ECD), require extremely pure gases because of their sensitivity. Carefully follow the recommendations of the column and detector manufacturer regarding gas supplies.
Practice, Practice, Practice
As a gas chromatographer, you will have the opportunity to work with your hands in operating and maintaining the instrumentation. Septa, columns, and glass sleeves need to be changed. Gas tanks and scrubbers get replaced, detectors are cleaned, and minor repairs are often performed by users. As with all equipment, there are tips and tricks that will help ensure successful maintenance and repairs.
Compression Fittings, Tubing, and Ferrules
You will be using compression fittings and ferrules in many situations. It is important that connections using compression fittings be made using good techniques or leaks can result. External to the instrument, the gas lines that connect it to the laboratory gas supply are typically connected using brass compression fittings and copper tubing or using stainless-steel tubing and fittings. For inertness and ruggedness, stainless steel is preferred, but it can be difficult to bend and work with. For ease of use, copper tubing and brass fittings are often used. In either case, be sure to use specially cleaned tubing obtained from a chemical or chromatographic supply vendor. Tubing from the hardware store will not be clean enough for GC. Do not mix copper and stainless-steel tubing and fittings; use one or the other. Do not use fittings and ferrules from different manufacturers on the same connection. The fittings are often threaded slightly differently or the ferrules may be different shapes. If used together, they may not fit well and are likely to leak.
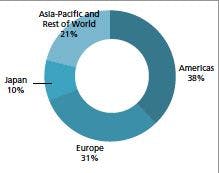
Figure 1 shows the assembly of a simple compression fitting on copper tubing using brass fittings. Cutting metal tubing and making proper compression fittings are skills that should be learned from an experienced practitioner or a training course; a few hints are provided here. When using a tubing cutter, do not cut all the way through the tube. Cutting all the way through the tube will constrict the end and might block the opening. It may distort the end, making it difficult to obtain a leak-free connection. Cut partially through the tubing wall and then make a clean break by placing both of your thumbs on either side of the cut and pushing outward. This technique is similar to cutting glass tubing. Check the end of the cut to ensure that it is clean; use a file to remove burrs or uneven areas.
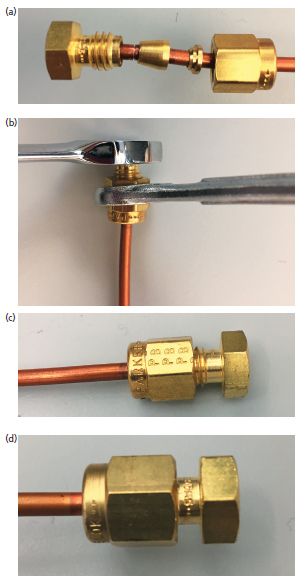
Figure 1: Brass compression fittings mounted on 1/8-in. o.d. copper tubing. (a) End cap, front ferrule, back ferrule, and nut loosely placed on the end of a tube. (b) Using open-ended wrenches to tighten the nut. Note the use of two open-ended wrenches to hold the nut and end cap. (c) Fully tightened end cap and nut but with fittings from two manufacturers. Note the exposed screw threads. (d) Correct fully tightened end cap and nut. Note no exposed screw threads.
Figure 2 shows a front ferrule mounted on a cleanly cut 1/8-in. o.d. copper tube. When working with compression fittings, be sure that the fitting and ferrule both slide easily onto the tubing. If not, use a file to smooth the rough area or make a fresh cut. Be sure the ferrule is pointed with the skinny end toward the end of the cut and not toward the nut. When tightening compression fittings, be sure that the tubing extends all the way onto the fitting, and use open-ended wrenches to tighten. Copper tubing should require not much more than hand tightening plus about one-quarter wrench turn. You should not crank down hard to ensure a tight fit. These same basic principles also apply to the many tubing connections in high performance liquid chromatography (HPLC), whether wrench or finger tightened.

Figure 2: Ferrule mounted on cleanly cut 1/8-in. o.d. copper tubing.
On most gas chromatographs, the column is connected to the inlet and detector using a compression fitting and a graphite or graphitized Vespel (Dupont) ferrule. Although column tubing is smaller and more delicate than copper or stainless steel, the same principles apply when working with column fittings. Number one: Do not overtighten; finger tight, plus one-quarter turn should be enough to ensure a leak free seal. For most applications in GC, the softer graphite ferrules are sufficient and easy to use for generating a good seal at both the inlet and detector ends of the column. For most GC–MS applications, harder ferrules are used. These may require a little more torque to tighten, but should still be tightened carefully to prevent leaks.
Finally, in all cases when working with capillary GC tubing and fittings, invest in a good electronic leak detector. A leak detector will save a lot of time and effort and allow you to easily check all new connections for leaks. Never use soap solutions, solvents, or the like for leak detection in capillary GC. These substances can diffuse into the tubing and cause contamination.
Trimming Columns
One of the first skills I learned as a new gas chromatographer was how to properly trim a column and prepare it for installation. You will do this often. As capillary columns age, impurities that degrade performance tend to deposit in the first few centimeters near the inlet. Simply trimming them off is a great way to revitalize a column. Properly cutting a fused-silica capillary column is a skill and requires practice. Many vendor websites provide instructions or videos that are a great place to start, and you can practice using an old column.
Trimming a capillary column is done following the same procedure as is used for cutting larger laboratory glass tubing, except the column is much smaller. Using a capillary scoring tool, usually a ceramic wafer, make a score (a mark) in the tubing surface. Then break the tubing quickly across the score, with the score pointing away from you. I flick it quickly with my finger. After the tubing is broken, use a magnifying glass to check the break. It should be clean and perpendicular. If it looks rough, make a new break. Rough spots will cause active sites that may adsorb analytes and cause reproducibility problems. Do not use scissors or other cutters to cut capillary tubing: That approach will always result in bad cuts. Close ups of good and bad cuts, taken with a cell-phone camera with a 30x microscope attachment obtained from a local toy store, are shown in Figure 3. If a bad cut was made, simply cut it again. Columns can generally sustain the shortening caused by several cuts before performance is affected.
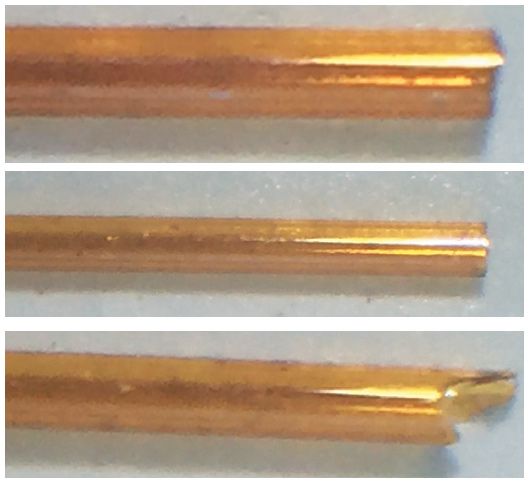
Figure 3: Good and bad column cuts. (a) Bad cut made by proper technique: Notice the rough end. (b) Good cut: The end is smooth. (c) Bad cut made by slicing all the way through the column with a ceramic cutting wafer. Note the very rough and broken end.
Column Installation
There are numerous guides and videos for column installation available. The best way to do this correctly is to follow the instructions provided by your instrument and column manufacturer. The exact instructions will vary by instrument. A few key points common to all instruments are presented here.
Wear safety glasses at all times when handling capillary columns. Coiled up is not the natural condition for a fused-silica capillary column. The ends can very easily snap out of the cage and hit you in the face. Fused silica is a glass so the ends are very sharp.
Trim the column after threading it through the ferrule. Otherwise, the sharp column end will scratch the inside of the ferrule bore and scrape graphite into the column. The end must be trimmed each time a column is passed through the hole in the ferrule. Figure 4 shows the end of a capillary column after passing it through the hole in a graphite ferrule. Note the black contamination at the tip.

Figure 4: The end of a capillary column after passing through a graphite ferrule. The black coloring at the tip is contamination from the ferrule.
On all GC systems the column ends are inserted into the inlet and detector. The connection must be leak-free and inert. On most systems, this is a blind installation. Be sure to know the insertion distance at the inlet and detector ends of the column. You cannot see the distance that the end of the column sticks out past the ferrule once the installation is complete. An old septum can be used as a holder to keep the column nut and ferrule at the correct distance for the column end. Figure 5 shows a septum attached behind the column nut at the inlet end of a gas chromatograph. The septum holds the nut in place during handling and installation, maintaining the correct column insertion distance into the inlet or detector. There is no harm from the septum remaining in the oven during use. Read the instrument manufacturer's instructions for insertion distance carefully.
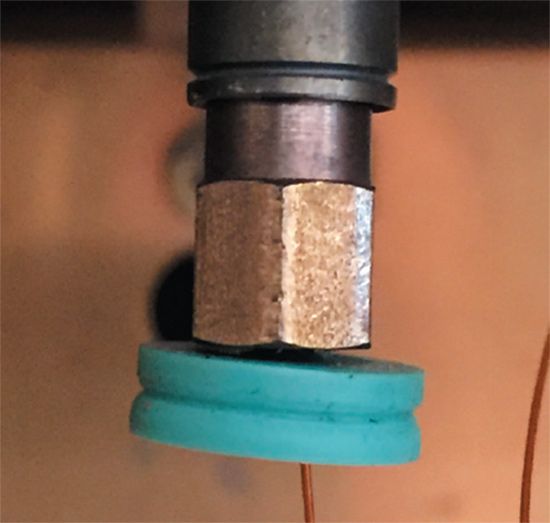
Figure 5: Septum threaded onto a capillary column behind the inlet installation nut.
When tightening the column nut, tighten no further than finger tight plus about one-quarter turn. If you need to tighten it more or torque it down hard, there is probably something wrong. If you then loosen the nut and see that the ferrule has deformed, it was too tight.
Be a Student of GC
GC is like chess-it can be learned in an afternoon but can take a lifetime to master. After nearly 30 years as a gas chromatographer, I am still discovering and learning new aspects of GC and separation science. For a new gas chromatographer, there is a large community of separation scientists and a huge volume of information available to assist you.
Ask Someone Who Knows
This is the best first step in troubleshooting, method development, and optimization. If more experienced gas chromatographers are close by in your organization, get to know them and ask for advice and assistance. Like other skills in chemistry, many of the laboratory skills needed for successful GC are not easily learned from books or webinars. As discussed below, these skills are often best learned personally from an expert and then practiced. Also, there are many local chromatography discussion groups across the United States and around the world that meet regularly, often with renowned speakers and in an informal setting for the discussion of real-world chromatography problems.
Get Some Good Books for Reference
For new chromatographers needing a quick introduction, I recommend the classic Basic Gas Chromatography, 2nd Edition, by McNair and Miller (2). Basic GC has been published since 1968 in numerous editions and has sold more than 200,000 copies worldwide. It is a short, inexpensive, hard-hitting introduction to what you need to know to get started in GC. For more thorough references, with more details on theory and applications, I recommend the comprehensive texts by Poole (3) and by Grob and Barry (4), which is considered by many as the bible for GC. I have all three of these on my bookshelf, and I refer to them often when troubleshooting, optimizing, and developing methods. As a professor and laboratory manager, these books are required reading for my students.
Get a Good Online Training System
There are several options for further training available online. I see these online training options as supplements to working personally with experienced chromatographers and reading. LCGC's ChromAcademy provides excellent breadth and depth of offerings, including articles, webinars, videos, and demonstrations (5). The materials are well developed and carefully reviewed. There are numerous sources for online training and information, including instrument vendors and additional specialized websites related to chromatography. When working with online information, be sure to confirm that the site is providing high quality, scientifically correct information.
Take a Short Course
In-person training courses still have an important role in educating new chromatographers. If you are purchasing a new instrument and the vendor offers hands-on training, take it. Even better, see if you can negotiate a seat in a training course as part of the purchase price. In my opinion, an in-person training course should be standard, especially when buying complex and expensive systems such as GC–MS, and definitely for GC–MS/MS, comprehensive GC (GCxGC), or GCxGC–MS. Live courses are usually taught by renowned experts in the field or by the vendor's technical experts on the specific instrumentation. You will often hear about tips, tricks, and techniques that are not in the manuals, textbooks, or website content. You will also have an opportunity to network with GC professionals at many experience levels. After 30 years of teaching and attending short courses, I still get new ideas from them.
Work Closely with Service Engineers and Technical Support
In chromatography, we are blessed with excellent service engineers and technical support from our vendors. Take advantage. If a service engineer visits your facility, do not just let the engineer into the laboratory and leave. Stay with the engineer, and watch and learn. Most routine maintenance and many repairs in GC performed under service contracts may be done in-house in a shorter time and at lower cost, saving time-consuming service visits for major problems. Also, service engineers usually are aware of many techniques specific to their instruments that do not always appear in the manuals. Many vendors have phone or online support. This type of support is also very useful. When describing your problem over the phone, be sure to provide as much detail as possible. Technical support is a little like your own doctor. You should provide accurate information about what was done with the system just before the problem occurred, even if it was your own mistake. They will not judge you, they will just help you get back up and running.
Many vendors now have extensive libraries of application notes, manuals, training materials, and guidebooks. This is a great and painless place to start when you have questions or a new method to develop. Simply go to the vendor's website, look for the section on GC and search for keywords that are appropriate to your problem. As a start, I suggest going to any of the instrument or column vendors and searching the phrase "GC troubleshooting." You will very likely be pointed to a useful troubleshooting guide. This search performed on LCGC's website produced more than 300 links, with a simple pictorial troubleshooting guide as the second item in the search results (6).
Conclusions
After more than 60 years, gas chromatography remains among the most widely used, versatile, and reliable instrumental techniques in use. As a staple instrument in many laboratories, GC is used by analysts of widely varying chemistry knowledge and experience. These tips and tricks are intended to help new users of GC obtain the best instrumental performance and to remind experienced users of some key aspects in gas chromatographic performance.
References
(1) D.W. Watson, LCGC North Am. 35(10), 776 (2017).
(2) H.M. McNair and J.M. Miller, Basic Gas Chromatography, 2nd Edition (John Wiley and Sons, New York, New York, 2009).
(3) C.F. Poole, Ed. Gas Chromatography, 1st Edition (Elsevier, Amsterdam, The Netherlands, 2012).
(4) R.L. Grob and E.F. Barry, Eds., Modern Practice of Gas Chromatography, 4th. Edition, (John Wiley and Sons, New York, New York, 2004).
(5) ChromAcademy, http://www.chromacademy.com/, accessed November 6, 2017.
(6) D.W. Watson, LCGC North Am. 34(10), 818 (2016).
ABOUT THE AUTHOR
Nicholas H. Snow

Nicholas H. Snow is Founding Endowed Professor in the Department of Chemistry and Biochemistry at Seton Hall University. He is also the university's Director of Research and Director of the Center for Academic Industry Partnership. During his 30 years as a chromatographer, he has published more than 60 refereed articles and book chapters and has given more than 200 presentations and short courses. He is interested in the fundamentals and applications of separation science, especially gas chromatography, sampling, and sample preparation for chemical analysis. His research group is very active, with ongoing projects using GC, GC–MS, two-dimensional GC, and extraction methods including headspace, liquid–liquid extraction, and solid-phase microextraction.
ABOUT THE COLUMN EDITOR
Jon. John V. Hinshaw

John V. Hinshaw GC Connections" editor John V. Hinshaw is a Senior Scientist at Serveron Corporation in Beaverton, Oregon, and a member of LCGC's editorial advisory board. Direct correspondence about this column to the author via e-mail: LCGCedit@ubm.com

Evaluating Body Odor Sampling Phases Prior to Analysis
April 23rd 2025Researchers leveraged the advantages of thermodesorption, followed by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS), to compare and assess a variety of sampling phases for body odor.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)