The Importance of Complete Overlapping of Analyte and Internal Standard Peaks in Eliminating Matrix Effects with Liquid Chromatography–Mass Spectrometry (LC–MS)
In the process of a liquid chromatography–tandem mass spectrometry (LC–MS/MS) assay development for two antimicrobial drugs using their stable isotope-labeled (SIL) analogues as internal standards to correct for matrix–ion suppression effects, an unusually large scatter of data was observed. A systematic observation revealed that the analytes and their SIL analogues (internal standards) were not completely coeluted; therefore, the analytes were not experiencing matrix effects similarly. When a column with lower resolution ability was used to achieve complete overlapping of the analyte and internal standard peaks, the scatter of LC–MS/MS data was minimized, indicating that the maximum correction of matrix effects by internal standards occurs when they are completely coeluted with the analytes. This study highlights the importance of ensuring complete overlapping of analyte and internal standard peaks in eliminating matrix effects when using SIL analogues as internal standards in LC–MS.
In liquid chromatography coupled to mass spectrometry (LC–MS), the ion suppression–enhancement effects because of the sample matrix can significantly reduce or enhance the analyte response (1–6). Ion suppression is more common than ion enhancement. When the analyte is coeluted with other compounds in the sample, the coeluted compounds compete with the analyte for either the total available charge or the available surface area of the droplet in the interface of the MS detector (6), leading to a diminished MS detector signal of the analyte ion. The ion suppression effect has been shown to reduce the accuracy of an assay by as much as 26% (5). Approaches such as removing impurities by sample cleanup have not always been successful in removing the ion suppression effects (7,8). Sample clean-up procedures, such as solid-phase extraction, removes compounds that are dissimilar in physicochemical properties, such as polarity and lipid solubility, from the sample to the analyte of interest. The compounds that are similar to the analyte of interest and thereby likely to be coeluted with the analyte are therefore not removed by the sample clean-up methods (9). As a result, sample cleanup is not necessarily a solution for matrix effects. In addition, it has been shown that even trace levels of compounds in mobile phase solvents can cause ion suppression (10). Therefore, preventing matrix effects in LC–MS is often unattainable. The only practical option to obtain LC–MS data that are not affected by matrix effects is to perform a correction for those matrix effects.
Correction for matrix effects is commonly achieved by using a specialized internal standard calibration procedure; the internal standard used must have almost exactly the same physicochemical properties as the analyte so that it will behave the same as the analyte both in the column and in the MS detector. This condition is commonly achieved in LC–MS by using a stable isotope-labelled (SIL) analogue of the analyte as the internal standard. This internal standard is often coeluted with the analyte but can be resolved from the analyte in LC–MS because of the slight difference in its mass from that of the analyte. As the SIL internal standard is expected to be eluted exactly at the retention time of the analyte, the presumption is that it experiences the same matrix/ion-suppression effects as the analyte in its passage through the MS detector. Therefore, by using the internal standard calibration with a coeluting SIL internal standard, the analyte peak response is usually corrected for the ion suppression matrix effects.
Because each analyte (eluted at a different retention time) is affected by different coeluted impurities, the concentration of each analyte needs to be corrected by using its own coeluting internal standard. Therefore, a coeluting SIL internal standard is required for the quantification of each analyte.
In the process of developing an LC–MS/MS method for the determination of N-(3-oxododecanoyl)-L-homoserine lactone (H) and fluconazole (F), using their stable isotope labelled analogues as internal standards, we observed an unusually large scatter in LC–MS data. This article describes the systematic investigation of the reason for this scatter and how the problem was resolved.
Experimental
Instrumentation
Compounds were separated using an Agilent 1100 LC binary pump and an Agilent 1100 autosampler. The columns used were as follows: A Zorbax Extend-C18, 3.5 μm 80 Å (2.1 x 50 mm) high performance liquid chromatography (HPLC) column from Agilent for method 1, and a Synergy 2 μm Fusion RP 100 Å (2.0 x 20 mm) HPLC column from Phenomenex for method 2. An API 3000 tandem mass spectrometer with a turbo ion spray interface and the software program Analyst 1.5 from Applied Biosystems were used for detection and quantification.
Materials
Fluconazole (F) and N-(3-oxododecanoyl)-L-homoserine lactone (H) were purchased from Sigma-Aldrich. The stable isotope analogue of N-(3-oxododecanoyl)-L-homoserine lactone, N-(12,12,12-d3-3-oxododecanoyl)-L-homoserine lactone (Hd), was synthesized by Dr. S.R. Chhabra at the Center for Biomolecular Sciences at the University of Nottingham in Nottingham, United Kingdom. The stable isotope analogue of fluconazole, fluconazole-d4 (Fd), was purchased from BOC Sciences. All solvents used were of HPLC grade.
Preparation of Samples and Standard Solutions
Stock solutions of analytes and deuterated analytes were prepared in acetonitrile and stored at -20 oC. Standard solutions for a six-point calibration curve were prepared to match analyte concentrations in 1000 times dilution of samples (as described below), using 100 μL of combined internal standard solution (300 μM N-(12,12,12-d3-3-oxododecanoyl)-L-homoserine lactone and 40 μM fluconazole-d4 in 10% acetonitrile aqueous solution), appropriate volumes of 100 μM fluconazole, appropriate volumes of 100 μM and 3.36 mM N-(3-oxododecanoyl)-L-homoserine lactone, and 10% acetonitrile to make the final volume up to 1000 μL. The concentrations of calibration standards were 10, 20, 30, 40, 50, and 60 μM N-(3-oxododecanoyl)-L-homoserine lactone; 1, 2, 4, 6, 8, and 10 μM fluconazole; 30 μM N-(12,12,12-d3-3-oxododecanoyl)-L-homoserine lactone (in all six standards) and 4 μM fluconazole-d4 (in all six standards) in 10% acetonitrile.
Preparation of Mobile Phase
Mobile phase A consisted of deionized water containing 0.1% (v/v) formic acid, and mobile phase B consisted of acetonitrile with 0.1% (v/v) formic acid. Both solutions were filtered through a 0.45 μm polytetrafluoroethene (PTFE) filter (Millipore) before they were used.
Chromatographic Conditions
Separation was carried out at an ambient temperature of approximately 25 oC. The flow rate was 200 μL/min with an injection volume of 10 μL. Following injection, analytes were separated using gradient elution: For method 1, mobile phase composition was changed from 10% B to 100% B during the first 15 min, then held at 100% B for 2 min before returning to 10% B from 17 to 20 min. The original composition of 10% B was maintained for the final 8 min prior to the next injection. For method 2, mobile phase composition was kept at 12% B for the first 6 min before increasing it from 12% B to 55% B during the next 3 min. Then, it was held at 55% B for the next 6 min before changing it to 100% over the next 2 min. Then, the mobile phase composition was held at 100% for 1 min before returning to 12% B over 2 min; the original composition of 12% B was maintained for the final 6 min to equilibrate the column prior to the next injection.
Mass Spectrometry Conditions
Multiple reaction monitoring (MRM) was used in positive ion mode. The transitions of 298 m/z ion to 102 m/z ion (for H) and 301 m/z ion to 102 m/z ion (for Hd); and 307 m/z ion to 238 m/z ion (for F) and 311 m/z ion to 242 m/z ion (for Fd) were monitored for each chromatographic run. The MS parameters were optimized for each analyte to obtain the highest sensitivity. The optimized values for H were: orifice-declustering potentials (DP) of 101 V, ring-focusing potentials (FP) of 370 V, collision energy (CE) of 19 V, and collision exit potential (CXP) of 8 V. The optimized values for F were: DP of 56 V, FP of 330 V, CE of 23 V, and CXP of 16 V.
An ion spray voltage (IS) of 5000 V and entrance potential (EP) of 10 V were used. Curtain gas (CUR), nebulizer gas (NEB), and the collision gas (CAD) flows were maintained at 12, 8, and 8 L/min, respectively. The temperature of the ion spray was maintained at 400 oC. A dwell time of 1000 ms was used for all transitions. Resolution of both Q1 and Q3 were 1 amu.
Results and Discussion
An unusually large scatter was observed with both H and F data obtained from the developed method (method 1). A systematic study was therefore carried out to identify the reasons behind this phenomenon. A combination standard containing H, Hd, F, and Fd was injected and LC–MS/MS was run six consecutive times using method 1, which was the method in use at the time. The upper chromatogram of Figure 1 shows peaks for H and F along with their coeluted internal standards Hd and Fd. The uppermost row of graphs in Figure 2 shows the individual peak areas of H and F, Hd and Fd, and the peak area ratio of the analyte and internal standard for H and F (all presented as percentage deviation from the third of the six runs). It is clear from Figure 2 that the internal standards did not correct for the differences in elution and detection conditions. The chromatograms, shown in the top of Figure 1, further reveal that there were slight differences in the retention times between analytes and internal standards. Differential retention times between analytes and their deuterated analogues have been previously observed (11,12) and attributed to the small differences in lipophilicity of the internal standard and the analyte because of deuteration. The difference is more pronounced with the F/Fd pair as Fd has four deuterium atoms whereas Hd has only three. Because of the differences in the retention times, the elution and detection conditions experienced by the analyte and the internal standard differ, and the resultant ion-suppression effects experienced by the two compounds are not similar. This explains the scatter in the data after correction with the internal standard (the ratio plot on the top right graph in Figure 2). The percent standard deviations for data in ratio plot 1 are 6.67% (H/Hd), and 26.2% (F/Fd), and those for ratio plot 2 are 1.35% (H/Hd), and 1.37% (F/Fd).
FIGURE 1: Chromatograms with scales adjusted to comparable peak sizes, showing the extent of coelution of fluconazole with its deuterated analog (early peaks), and homoserine lactone with its deuterated analog (late peaks). (a) Chromatogram is with method-1 and (b) chromatogram is with method-2. (a) Chromatogram shows that the (deuterated) internal standards have slightly longer retention times than the analytes F and H.
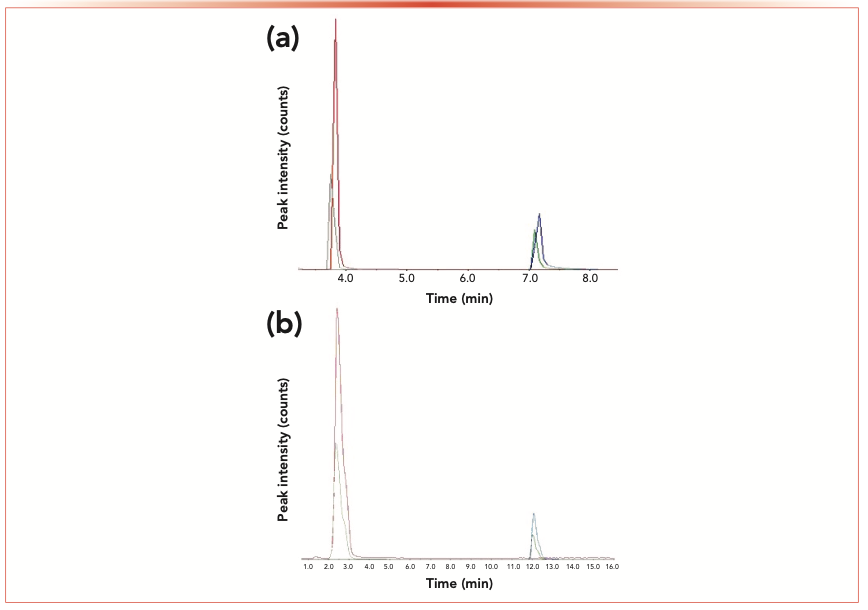
FIGURE 2: Note: x-axis label is successive chromatogram number and y-axis label is percentage deviation from 3rd chromatogram. Figures (a) and (b) show percentage deviations of peak areas from the third chromatogram of six successive chromatograms run using identical injections of a standard solution containing H (Homoserine Lactone), Hd (deuterated Homoserine Lactone–internal standard), F (Fluconazole) and Fd (deuterated Fluconazole–internal standard), using method-1. Figures (d) and (e) show the same using method-2. Figures (c) and (f) show percentage deviations of peak area ratios of analyte/internal standard for method-1 and method-2 respectively. The first bar in each pair of bars in all figures are for H or Hd and the second for F or Fd. The percent standard deviations for data in ratio plot 1 (c) are: 6.67% (H/Hd), and 26.2% (F/Fd), and those for ratio plot 2 (f) are: 1.35% (H/Hd), and 1.37% (F/Fd).
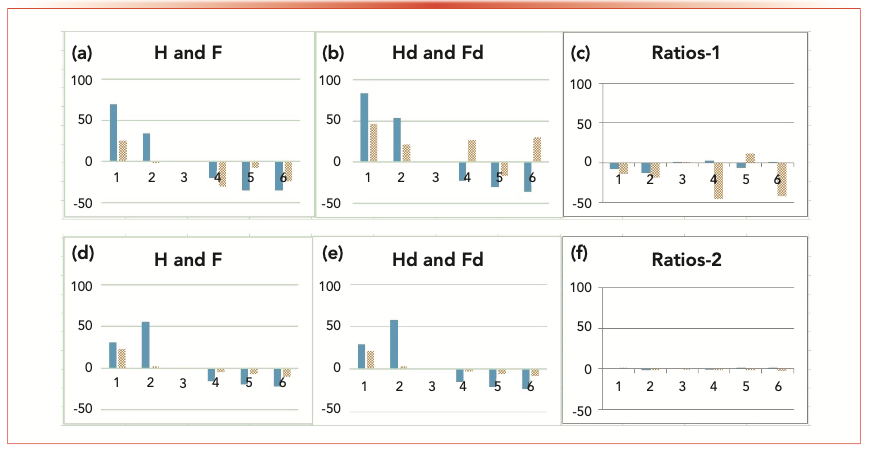
To force the two compounds to be coeluted, various gradients were investigated. However, the differences in lipophilicities and the resolution capabilities of the column were too high to achieve coelution simply by changing the elution conditions. Therefore, a column with lower resolution capacity was used to promote the overlap but still to achieve similar retention of both analytes. By using an appropriate gradient with the new column (method 2), we achieved better overlap of peaks (bottom chromatogram in Figure 1). The bottom row of graphs in Figure 2 shows the effect of peak overlapping: Although there is observable scatter in individual peak areas in the first two graphs in the second row, there is insignificant scatter in the third graph (the ratio of analyte to internal standard), indicating the intended function of the internal standard. The extent of overlap in Figure 1 (top vs. bottom) is suggested as the direct cause of the extent of scatter in the last column of Figure 2 (top vs. bottom). Thus, it is clear that despite the seemingly very small difference in peak overlap, its impact on the accuracy and precision of the data is significant.
Therefore, it is advisable to ensure precise coelution by periodic surveillance of chromatograms obtained in routine LC–MS methods as well as in method development because slight changes in the column and in the eluents and solvents may affect the extent of coelution. It is also recommended to examine and ensure the linearity of analyte and the internal standard responses, within the concentration ranges expected, when using the analyte–internal standard response ratio for calibrations (13).
To obtain a significant mass resolution and to prevent cross-talk, the masses between the analyte and the internal standard must differ at least by 3 amu (12). As observed in our study, replacing the three H atoms by deuterium can change the physicochemical properties, likely lipophilicity, of the molecule significantly.
The problem of differential lipophilicity, and its effect on the change in retention, can be minimized by using alternative (to deuterated) stable isotope-labelled analogues such as C-13, N-15, or O-17 as internal standards (11,12). As analysts who develop LC– MS methods are well aware, sourcing a SIL internal standard is a challenge that is often expensive. The most common types of SIL internal standards available are deuterated analogues. The only other effective alternative to using SIL internal standards, to correct for matrix effects in LC–MS, is to use the standard addition with internal standardization (14).
Conclusion
The effects of incomplete coelution of the analyte and SIL internal standard on the scatter and the accuracy of the LC–MS data was studied. Compound deuteration affects the retention of analytes on reversed phase chromatography, causing the analyte and its deuterated analogue to separate slightly. This separation lead to incomplete coelution of the analyte and SIL internal standard, and consequently differential matrix effects on the analyte and the internal standard, giving rise to scattered and inaccurate results with internal standard calibration. Using a column with reduced resolution to achieve coelution of the analyte and the deuterated internal standard proved to be an effective method in overcoming the problem.
Acknowledgments
This study was funded by an International Association for Dental Research (IADR)-GlaxoSmithKline Innovation in Oral Care Award 2016 awarded to Dr. H.M.H.N. Bandara.
References
(1) P. Kebarle and L. Tang, Anal. Chem. 65, 972A–986A (1993).
(2) B.K. Matuszewski, M.L. Constanzer, and C.M. Chavez-Eng, Anal. Chem. 75, 3019–3030 (2003).
(3) R. King, R. Bonfiglio, C. Fernandez- Metzler, C. Miller-Stein, and T.J. Olah, J. Am. Soc. Mass Spectrom. 11, 942–950 (2000).
(4) P.J. Taylor, Clin. Biochem. 38, 328–334 (2005).
(5) D.L. Buhrman, P.I. Price, and P.J. Rudewicz, J. Am. Soc. Mass Spectrom. 7, 1099–1105 (1996).
(6) R.K. Boyd, C. Basic, and R.A. Bethem, Trace Quantitative Analysis by Mass Spectrometry (John Wiley, West Sussex, England, 2008).
(7) M. Ivano, V. Viette, F. Badoud, M. Fathi, M. Saugy, S. Rudaz, and J.-L. Veuthey, J. Chromatogr. A 1217, 4071–4078 (2010).
(8) F. Gosetti, E. Mazzucco, D. Zampieri, and M.C. Gennaro, J. Chromatogr. A 1217, 3929–3937 (2010).
(9) A.K. Hewavitharana, J. Chromatogr. A 1218, 359–361 (2011).
(10) H.M.D.R. Herath, P.N. Shaw, P. Cabot, and A.K. Hewavitharana, Rapid Commun. Mass Spectrom. 24, 1502–1506 (2010).
(11) S. Wang, M. Cyronak, and E.J. Yang, Pharm. Biomed. Anal. 43, 701–707 (2007).
(12) E. Stokvis, H. Rosing, and J.H. Beijnen, Rapid Commun. Mass Spectrom. 19, 401–407 (2005).
(13) A.K. Hewavitharana, Crit. Rev. Anal. Chem. 39, 272–275 (2009).
(14) A.K. Hewavitharana, N.S. Abu Kassim, and P.N. Shaw, J. Chromatogr. A 1553, 101–107 (2018).
Amitha K. Hewavitharana and P. Nicholas Shaw are with the School of Pharmacy at the University of Queensland in Queensland, Australia. H.D.C. Smyth is with the College of Pharmacy at the University of Texas at Austin in Austin, Texas. L.P. Samaranayake is with the College of Dental Medicine at the University of Sharjah in Sharjah, United Arab Emirates. H.M.H.N. Bandara is with the Bristol Dental School at the University of Bristol in Bristol, United Kingdom. Direct correspondence to: a.hewavitharana@pharmacy.uq.edu.au.

Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.














