Synthesis and Applications of BEH Particles in Liquid Chromatography
Special Issues
How ethylene-bridged hybrid inorganic–organic (BEH) particles are prepared and how their special qualities can be used to improve separations
Ethylene-bridged hybrid inorganic–organic (BEH) particle technology has allowed for the improvement in separation performance for a range of separation modes, including reversed-phase, hydrophilic interaction, and size-exclusion chromatography. In this report, the process of preparing BEH particles, the characterization of these materials, and their use for unique chromatographic applications are explored.
The development of column packing materials is a competitive field that is the focus of many industrial research and development (R&D) groups worldwide. The result of these efforts is the commercialization each year of new families of separation columns — all claiming to have improvements over the prior technologies. End users may wonder why we need so many different column brands and phases.
The question asked in an industrial R&D setting is: How can a new family of column packings be differentiated from others in the marketplace? To achieve a successful product, R&D efforts need to be focused on user needs. As shown in Figure 1, there are a number of user considerations for new separation columns. A new column family may try to differentiate on one or more of these user needs.

Figure 1
Because user needs change over time, it is critical for an R&D team to be able to feel the pulse of these changing requirements. In the 1990s, users were struggling with column reproducibility and as a result, industrial R&D efforts focused on improving the reproducibility and purity of porous silica packing materials (1). At the turn of the century, a major user need was increased selectivity options and research groups addressed that need with the development of new stationary phases (2).
Our exploration of hybrid inorganic–organic materials (denoted hybrids) started in the mid-1990s as a way to address the needs of scientists using reversed-phase chromatography for improved selectivity, efficiency, resolution, peak shape, stability, and reproducibility (3). We found hybrid particles to be high performance materials that are well suited to meet these needs. This technology has expanded into the preparation of new materials to address user requirements in hydrophilic interaction (HILIC) and size-exclusion chromatography (SEC). In the following sections, we will detail how hybrid particles are prepared and how their unique qualities can be used to improve separations.
Development of Hybrid Particles
Hybrid materials have a mixed composition of silica and organosiloxanes. Our initial research into hybrid particles focused on the use of methylsiloxanes, and these products were commercialized in 1999 as the XTerra family of reversed-phase columns (4,5). These columns demonstrated improved chemical stability and reduced silanol activity over silica-based reversed-phase columns.
As hybrid columns started to be adopted, our research focused on preparing novel hybrid formulations and exploring new chromatographic applications for these materials. Early in this research, we determined that improved performance can be achieved with a new hybrid formulation based on an ethylene-bridged hybrid group. This became the foundation of our BEH particle technology and the basis of a number of commercial products since the initial introduction in 2004 (6,7).
The BEH particle (Figure 2) is formed in a patented, multistep manufacturing process, shown in Figure 3 (8,9). In the first step of this process, two high-purity monomers, tetraethoxysilane and 1,2-bis(triethoxysilyl)ethane, are co-condensed in a 4:1 molar ratio to form a water-immiscible polyethoxyoligosilane polymer (denoted PEOS). In the next step, the PEOS is emulsified in water (o/w emulsion) to form highly spherical oil droplets. Surfactants and high shear mixers are employed for control of droplet size. Upon addition of a catalyst, the condensation reaction completes to yield highly spherical, porous hybrid particles. The pore volume of BEH materials is controlled in this step through the use of chemical additives called porogens. Modification of porogen concentration can be used in this process step to produce new materials that differ in pore volume over a wide range (0.3–1.6 cm3 /g). BEH particles used in reversed-phase and HILIC separations have a 0.7-cm3 /g pore volume. In contrast, BEH particles used in SEC have a 1.3-cm3 /g pore volume.

Figure 2
Next, we modify the surface area and pore diameter of these materials with a pore ripening step (8). This step exposes the particles to elevated temperatures and pH to allow for increased pore diameters and decreased surface areas. By changing the conditions for this process step we have been able to produce hybrid particles with average pore diameters that range between 40 and 760 Å. The pore diameter of the BEH particles is tailored for specific separations. The BEH particles used in small molecule reversed-phase and HILIC separations have a 130-Å pore diameter; materials used for peptide and protein reversed-phase separations have a pore diameter of 300 Å; and materials used for size-exclusion chromatography have 125-Å or 200-Å pore diameters.
Additional steps shown in Figure 3 include particle sizing, bonding, and column packing. These parameters are fully optimized for individual applications. Following this process, hybrid particles have been produced with particle sizes of 1.7–10 µm.
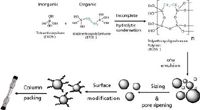
Figure 3
While the process of producing hybrid particles is multistep, this approach is well understood and has been fully scaled to the multikilogram level. During the initial development program, we determined how every step of the process impacted the critical design parameters. We found that using separate reaction steps to control critical design parameters ensures a high level of batch-to-batch reproducibility. This process also is useful for tailoring new materials for specific applications (10). For example, high resolution, faster SEC separations can be achieved using 1.7-µm BEH particles that have increased pore volume. Alternatively, wide-pore BEH particles that are used for reversed-phase separations of proteins and peptides are prepared using the same BEH precursors as those used for analytical small molecule separations, but with a different pore ripening step. Table I details some physical parameters of these tailored BEH particles.

Table I: Selected physical parameters of BEH technology columns
Understanding BEH Particle Technology
The BEH particles have many similarities to high-purity porous silica. For example, these materials have surface silanols that can be derivatized with silanes to yield a variety of different reversed-phase materials (such as, C18, C8, phenyl-hexyl, and fluoro-phenyl). These similarities allow most separations using BEH columns to use a similar method development as traditional silica columns.
The BEH materials differ from silica because ethylene-bridged hybrid groups are homogeneously distributed throughout the particle. These internal and surface hybrid groups modify the chemical properties of these materials in a number of ways that are advantageous for use in separations. This includes improved chemical stability and reduced silanol activity over silica columns.
Acid Stability of Bonded Phases on Hybrid Particles
When developing a new chromatographic stationary phase, it is important to assess chemical stability. The use of accelerated stability tests allows one to determine how extreme operating conditions impact the efficiency or retentivity of a column. Loss in efficiency can be an indicator of poor mechanical stability or the dissolution of the packing material. Changes in retentivity are often indicative of bonded-phase hydrolysis.
When reversed-phase columns are evaluated using an accelerated acid stability test, the stability concern is bonded-phase hydrolysis and not particle dissolution. In these tests, we monitor the loss in retention for neutral analytes (11). Stability under acidic conditions is bonded-phase dependent, and not a function of the base particle chemical formulation. As a result, similarly bonded hybrid and silica reversed-phase columns have comparable stability results. Improving acidic stability requires improvements in bonded phase technologies. The use of trifunctional bondings, for example those based on octadecyltrichlorosilane, allows for improved acid stability over monofunctional bondings, such as those based on octadecyldimethylchlorosilane. However, trifunctional bonded phases have historically suffered from poor reproducibility. Fully optimizing trifunctional bondings was initially a challenge. Our prelaunch prototypes consisted of more than 130 different experiments over a two-year timeframe. Each material had to be bonded, optimally packed, and fully evaluated using a series of tests (for example, isocratic and gradient; pH 3, 7, or 10; UV or mass spectrometry [MS] detection; and van Deemter analysis). In order to process this large dataset, we used similar design of experiments (DoE) and quality by design (QbD) methodologies that many of our customers use. The end result of this was a new approach to trifunctional bonding and endcapping that yielded a high level of reproducibility and improved low pH stability of our packing materials. This advanced bonding technology is an important part of BEH particle technology.
Base Stability of Bonded Phases on Hybrid Particles
Chromatographers using reversed-phase chromatography sometimes prefer to work under high-pH conditions for reasons of increased retention of basic compounds, increased compound stability, improved selectivity, or the ability to use volatile MS-compatible buffers like ammonia. One of the most notable attributes of BEH columns is stability when used under basic conditions (7). The BEH particles have increased chemical stability at elevated pH because of the hydrophobicity and internal cross-linking of the ethylene-bridged hybrid group. A standard test for base stability challenges reversed-phase columns at elevated pH (10) and temperatures (50 °C). The traditional failure mode of silica-based reversed-phase columns under these conditions is catastrophic loss of column efficiency because of particle dissolution (12,13). Visual inspection of silica-based reversed-phase columns after testing often reveals the formation of voids. The BEH columns are stable under these conditions. To demonstrate a clear change in performance for BEH columns, this base stability test needed to be modified to further increase the pH to 12.3 using 0.02 N sodium hydroxide. Such demanding conditions were only used to compare hybrid columns, as silica columns fail with a small number of injections. For hybrid columns, failure generally occurs because of a loss of retention resulting from bonded phase hydrolysis, rather than a loss in efficiency because of particle dissolution. It is important to have strong bonded-phase attachment to the particle to match the outstanding base stability of hybrid particles. The use of exhaustively endcapped, trifunctionally bonded hybrid columns provides improved retention stability under these demanding high-pH conditions.
Hybrid Particles Used in Reversed-Phase Chromatography
This extreme pH stability can be used to optimize reversed-phase selectivity because changes in pH impact the ionization of both analytes and the particle surface. As shown in Figure 4 for an Acquity UPLC BEH C18 column, a diverse set of analytes can be separated under reversed-phase conditions using pH 3 or pH 10 gradients. For the pH 3 separations, acidic analytes (shown in red) are normally unionized and basic analytes (shown in blue) are ionized. Ionized analytes have lower retention times than their unionized forms. At pH 10, the basic analytes are generally in an unionized state and are more retained, while acidic analytes are ionized and less retained. As expected, neutral analytes are not greatly affected by changes in mobile-phase pH.
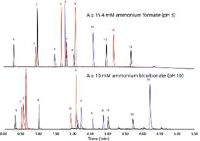
Figure 4
Hybrid Particles Used in HILIC
Modifying the mobile-phase pH in HILIC separations also can be used to change selectivity (14). However, because of the high organic mobile phases required in HILIC separations, other considerations are needed to fully understand pH impacts on retention times, for example the polarity of the ionized molecule and stationary phase ionization. One major advantage of using high pH in HILIC separations is MS response. By having analytes in an ionized form we often observe sharp increases in electrospray MS signal intensities. These can be compared using the ratio of peak areas from pH 9 and pH 3 separations, which have been shown to have a 0.7–35-fold improvement at pH 9 (15).
An example of using a hybrid column under HILIC conditions at elevated pH would be carbohydrate separations using an Acquity UPLC BEH Amide column. Carbohydrate analysis can be challenging and is often impacted by slow interconversion of reducing sugars anomers, impacting the speed, resolution, and unreliable quantitation. The interconversion of α/β anomers can be accelerated at high temperatures or when exposed to elevated pH. Although traditional amino bonded silica-phases allow for rapid anomerization because of the localized surface alkalinity, these phases suffer from poor chemical stability because of autocatalyzed bonded phase hydrolysis. The reaction of reducing sugars with the amino group of the stationary phase also may lead to decreases in peak areas (16–19).
BEH Amide columns have greatly improved particle and bonded phase chemical stability compared to amino bonded silica-columns. Accelerated base stability tests performed on BEH Amide columns (0.05% triethylamine, 80% acetone, pH 10.35, 90 °C) showed minimal loss in retention over 2000 injections. This improved chemical stability at both elevated pH and temperature (Figure 5) allows for rapid interconversion of reducing sugar anomers (20).

Figure 5
Chromatographic Impact of Silanol Activity
Underivatized (unbonded) silanol groups on the surface of packing materials can impact the ionic properties as well as the retention of ionizable analytes for reversed-phase and HILIC separations because of ion-exchange or ion-exclusion interactions. The acidity of these silanol groups is impacted by the composition of the material. Impurities can often result in increased silanol acidity, which can negatively impact chromatographic performance. The need for improved reproducibility and peak shape for basic analytes was a major user need that drove the research into higher purity silica in the 1990s. Although the advent of higher purity silica led to improved peak shapes for basic analytes, there was still a need for further improvement.
Silanol acidity of silica and hybrid packing materials has been examined for underivatized and C18-bonded particles by measuring retention changes of an ionized analyte (bretylium tosylate or LiNO3) under different mobile-phase conditions (pH 2–11 and from water to 80% acetonitrile) (21,22). These studies showed a higher ionization pH for hybrid particles over silica (pH 10 and pH 7, respectively). Because hybrid materials are a mixture of silica and organosiloxane groups, the acidity of the surface silanol groups is not expected to be the same as that of silica.
Hybrid Particles Used in SEC
This lower silanol acidity for hybrids compared to silica allows for improved peak shape for basic analytes under reversed-phase conditions. Aqueous SEC is another application where performance may be negatively affected by silanol activity (16,23,24). For optimal SEC separations one wants to have the highest possible particle porosity and no secondary interactions. To limit secondary interactions because of acidic silanols of silica-based SEC particles, hydrophilic diol bonded phases are often used. A thick coating of the diol phase, which can greatly decrease the porosity of these materials, is required to appropriately shield the electrostatic charge of the acidic silanols from the analytes. Even with optimal diol bondings the effects of surface silanols are still observed, requiring the use of mobile phases with high ionic strength to minimize ionic retention.
One method to test for acidic silanols on SEC columns is to monitor the chromatographic behavior of the basic protein lysozyme (pI ~11) at pH 7 using mobile phases containing different phosphate buffer concentrations. When comparing silica-based SEC columns with the BEH SEC column, as shown in Figure 6, the results are clear. With low ionic strength mobile phases (10–25 mM) the BEH column exhibits less adsorption of the basic protein lysozyme, when compared to a commercially available silica SEC column.

Figure 6
Charged Surface Hybrids
As noted above, user needs continue to change. Today, there are still difficult problems facing scientists that require new column solutions, including additional selectivity options and improved LC–MS separation solutions. Many LC–MS users experience problems with commercially available reversed-phase columns that are attributable to poor performance with MS-compatible mobile phases (for example, formic acid, acetic acid, or ammonia). Specific problems include slow equilibration and poor peak shape for basic analytes at low pH (25–27). These issues have been linked to a variation in the surface charge of the packing materials, which is amplified by the use of low-ionic-strength mobile phases.
We undertook a two-year research program to better understand these issues (28). Slight changes in surface charge because of mobile phase contaminants or impurities left from the particle synthesis or bonding process can impact chromatographic performance with low ionic strength mobile phases. Such issues are masked with higher-ionic-strength mobile phases or ion-pairing reagents (22,29, 30). These effects impact both hybrid and silica materials and are evident on most modern high-purity stationary phases.
Rather than continue research controlling impurity levels on the surface of these particles, we explored a different path in controlling surface charge through surface modification using ionizable silanes. The end result of this research was the development of a new family of columns based on BEH particle technology, called Charged Surface Hybrids (CSH) (28). In this approach, BEH particles were surface- modified with a low concentration of a weakly basic ionizable silane, followed by bonding of the primary phase (for example, C18) and endcapping. The optimal surface concentration of the ionizable silane groups is more than an order of magnitude lower than the primary bonded phase. The weakly basic silane group is protonated at a low pH and is neutral above pH 7.
CSH columns are available in a 1.7-µm particle size as Acquity UPLC CSH columns as well as 2.5–5 µm particle sizes as part of the XSelect CSH column family. These columns are available in C18, phenyl-hexyl, and fluoro-phenyl bonded phases, allowing for a large breadth of selectivity options.
CSH columns have notable improvements in peak shape for basic analytes with low-ionic-strength mobile phases compared to other C18 columns. The CSH C18 column maintains nearly linear-isotherm behavior for amitriptyline at mass loads that approach those used in purification applications. This improvement in peak shape for basic analytes also is realized in gradient separations, and allows for notable improvements in peak capacity for basic analytes. As shown in Figure 7, a 1.7-µm BEH C18 column has poor peak shapes for the bases metoprolol and amitriptyline when used with a 0.1% formic acid mobile phase because of mass overloading (31). In contrast, the 1.7-µm CSH C18 column does not suffer this limitation, so that the peak capacities for these bases are similar to those of the neutral analytes prednisone and caffeine. The CSH C18 column can take full advantage of the efficiency gains from using sub-2-µm packing materials when using formic acid mobile phases for basic analytes (32,33).
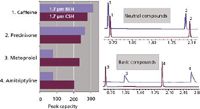
Figure 7
Because CSH particles are based on BEH particle technology, they have been designed to have outstanding chemical stability when exposed to acidic and basic mobile phases, and are well suited for LC–MS separations. These columns are highly reproducible (28), and because the level of ionizable silane group is low, the impact on traditional reversed-phase performance is minimal.
Conclusions
BEH particle technology has been developed to meet changing user needs. This process allows for excellent manufacturing control of material properties, as well as the ability to tailor particle properties for specific applications. Through modification of particle porosity and pore diameters, BEH particles can be prepared and used for a diverse set of applications, including reversed-phase, HILIC, and size-exclusion chromatography of small and large molecules. Hybrid particles offer unique chemical properties, including lower silanol activity and improved high pH stability over silica, allowing for dramatic improvements in these separations.
Kevin Wyndham is a manager of a materials research group at Waters Corporation, Milford, Massachusetts. Thomas Walter is the senior director of chromatography chemistry R&D at Waters Corporation. Pamela Iraneta is a manager of a chromatographic research group at Waters Corporation. Bonnie Alden is a senior research scientist in a chromatographic research group at Waters Corporation. Edouard Bouvier is a manager of a chromatographic research group at Waters Corporation. Christopher Hudalla is a principal applications chemist in the chemistry applications group at Waters Corporation. Nicole Lawrence is a senior research chemist in a materials research group at Waters Corporation. Daniel Walsh is a senior research scientist at Waters Corporation. Please direct correspondence to: kwyndham@waters.com.
References
(1) U.D. Neue, E. Serowik, P. Iraneta, B.A. Alden, and T.H. Walter, J. Chromatogr. A 849(1), 87–100 (1999).
(2) J.E. O'Gara, B.A. Alden, T.H. Walter, J.S. Petersen, C.L. Niederlaender, and U.D. Neue, Anal. Chem. 67(20), 3809–3813 (1995).
(3) P.D. McDonald, in The Quest for Ultra Performance in Liquid Chromatography, www.waters.com, PN 715002098, 2009 June.
(4) Y.F. Cheng, T.H. Walter, Z. Lu, P. Iraneta, B.A. Alden, C. Gendreau, U.D. Neue, J.M. Grassi, J.L. Carmody, J.E. O'Gara, and R.P. Fisk, LCGC N. Amer. 18(11), 1162–1172 (2000).
(5) U.D. Neue, T.H. Walter, B.A. Alden, Z. Jiang, R.P. Fisk, J.T. Cook, K.H. Glose, J.L. Cormody, J.M. Grassi, Y.F. Cheng, Z. Lu, and R.J. Crowley, Am. Lab. 31(22), 36–39 (1999).
(6) J.E. O'Gara and K.D. Wyndham, J. Liq. Chromatog. Rel. Tech. 29(7–8), 1025–1045 (2006).
(7) K.D. Wyndham, J.E. O'Gara, T.H. Walter, K.H. Glose, N.L. Lawrence, B.A. Alden, G.S. Izzo, C.J. Hudalla, and P.C. Iraneta, Anal. Chem. 75(24), 6781–6788 (2003).
(8) N.L. Lawrence, K.D. Wyndham, K.H. Glose, J.T. Cook, D.W. Brousmiche, P.C. Iraneta, B.A. Alden, C.A. Boissel, and T.H. Walter, Mater. Res. Soc. Symp. Proc. 1007, 41–47 (2008).
(9) US Patents No. 6,686,035 B2, 7,223,473 B2, and 7,919,177 B2.
(10) M. Jacoby, Chem. Eng. News 86(17), 17–23 (2008).
(11) B.C. Trammell, C.A. Boissel, C. Carignan, D.J. O'Shea, C.J. Hudalla, U.D. Neue, and P.C. Iraneta, J. Chromatogr. A 1060(1–2), 153–163 (2004).
(12) J.J. Kirkland, M.A. van Straten, and H.A. Claessens, J. Chromatogr. A 691(1–2), 3–19 (1995).
(13) K.D. Wyndham, T.H. Walter, P.C. Iraneta, U.D. Neue, P.D. McDonald, D. Morrison, and M. Baynham, in A Review of Waters Hybrid Particle Technology, Part 2, Ethylene-Bridged [BEH TechnologyTM] Hybrids and Their Use in Liquid Chromatography, Waters white paper 720001159EN. 2005 June.
(14) E.S. Grumbach and K.J. Fountain, in Comprehensive Guide to HILIC, Hydrophilic Interaction Chromatography, www.waters.com PN 715002531, 2010 June.
(15) K.J. Fountain, J. Xu, D.M. Diehl, and D. Morrison, J. Sep. Sci. 33(6–7), 740–751 (2010).
(16) U.D. Neue, in HPLC Columns Theory, Technology and Practice (John Wiley & Sons, Hoboken, New Jersey, 1997), pp. 140–163.
(17) M. Liu, E.X. Chen, R. Ji, and D. Semin, J. Chromatogr. A 1188(2), 255–263 (2008).
(18) L. Nováková, I. Kaufmannová, and R. Jánská, J. Sep. Sci. 33(6-7), 765–772 (2010).
(19) C. Hudalla, J. Cook, M. Dion, P. Iraneta, K. Jenkins, P. Smith, D. Walsh, and K. Wyndham, "UPLC Analysis of Carbohydrates: Applications for Saccharide Analysis in Food and Beverage Products and Pharmaceutical Excipients," presented at AOAC International Annual Meeting, Philadelphia, Pennsylvania, 2009.
(20) U.D. Neue, C.J. Hudalla, and P.C. Iraneta, J. Sep. Sci. 33(6–7), 838–840 (2010).
(21) U.D. Neue, C.H. Phoebe, K. Tran, Y.-F. Cheng, and Z. Lu, J. Chromatogr. A 925(1–2), 49–67 (2001).
(22) A. Méndez, E. Bosch, M. Rosés, and U.D. Neue, J. Chromatogr. A 986(1), 33–44 (2003).
(23) A. Striegel, W.W. Yau, J.J. Kirkland, and D.D. Bly, in Modern Size-Exclusion Liquid Chromatography: Practice of Gel Permeation and Gel Filtration Chromatography, 2nd Edition, (John Wiley & Sons, Hoboken, New Jersey, 2009).
(24) K.K. Unger, in Silica as Packing in Size-Exclusion Chromatography, Porous Silica, Its Properties and Use as Support in Column Liquid Chromatography, (Elsevier, Amsterdam, Netherlands, 1979), Vol. 16, pp. 271–289.
(25) D.V. McCalley, J. Chromatogr. A 1075(1–2), 57–64 (2005).
(26) J.J. Gilroy, J.W. Dolan, and L.R. Snyder, J. Chromatogr. A 1000(1–2), 757–778 (2003).
(27) D.H. Marchand, L.A. Williams, J.W. Dolan, and L.R. Snyder, J. Chromatogr. A 1015(1–2), 53–64 (2003).
(28) P.C. Iraneta, K.D. Wyndham, D.R. McCabe, and T.H. Walter, in A Review of Waters Hybrid Particle Technology, Part 3, Charge Surface Hybrid (CSH) Technology and Its Use in Liquid Chromatography, Waters white paper 720003929EN. 2010 June.
(29) D.V. McCalley, J. Sep. Sci. 26(3–4), 187–200 (2003).
(30) E. Loeser and P. Drumm, Anal. Chem. 79(14), 5382–5391 (2007).
(31) D.V. McCalley, Anal. Chem. 78, 2532 (2006).
(32) K.J. Fountain and H.B. Hewitson, LCGC N. Amer. 29(2), 56–57 (2011).
(33) K.J. Fountain, H.B. Hewitson, P.C. Iraneta, and D. Morrison, LCGC N. Amer. 28(10), 13–18 (2010).

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.









