A New Generation of Silica-Based Monoliths HPLC Columns with Improved Performance
Special Issues
Monolithic columns feature both high column efficiency and high column permeability. This article discusses their preparation, applications, and future prospects.
With the advent of the second generation of commercial silica monolith columns, liquid chromatographers now have an alternative to the sub-2-µm and core–shell packings for high-throughput, high-efficiency separations. In this paper, the preparation of the new-generation silica-based monolithic high performance liquid chromatography columns is described in detail and the first- and second-generation columns are compared based on physical characteristics, with application examples given. The future prospects for silica-based monoliths also are explored.
Monolithic high performance liquid chromatography (HPLC) columns are made of one single piece of either porous silica or organic polymer. These columns have a unique chromatographic feature: high column efficiency and high column permeability at the same time. Therefore, there is an increased interest in these materials, driven by the never-ending demand for faster and more efficient HPLC methods. Both types of monolithic columns are now commercially available and under intensive investigations. Their chromatographic properties and applications have been described in several recent reviews (1–11). Two features primarily differentiate the silica-based monoliths from the organic polymeric-based monoliths: pH stability and separation efficiency (plate count per meter = N/m). While pH stability is definitely better for organic polymeric monoliths (pH 1–12), separation efficiency is undoubtedly better for silica monoliths (N/m up to 200,000). This article will focus on the latest advancements in the development of silica-based monoliths while organic polymeric monoliths are reviewed by others (7–12).
The first silica-based monolithic HPLC column (Chromolith, Merck Millipore) became commercially available in 2000 and attracted a lot of attention because of its novelty. This type of HPLC column consists of a porous silica rod that is encapsulated in a mechanically strong and solvent-resistant PEEK polymer and equipped with low-volume endfittings. The high porosity of silica-based monoliths (total porosity > 80%) is caused by macro- or through-pores that offer significant advantages compared to classical particle-packed columns — for example, low column back pressures, operation with higher flow rates, faster analysis, and direct applications of "dirty" samples without prior sample preparation (2). However, in the last 5 years, particle-packed columns filled with either sub-2-µm silica particles or with superficially porous particles (that is, core–shell particles) have been developed in parallel with the monolithic columns (13,14). As chromatographic theory has predicted, separation efficiency is inversely proportional to the particle diameter. This is why columns filled with smaller porous particles show very high separation efficiencies. Since the beginning of HPLC in the 1970s, the predominant particle size has constantly decreased from 10 µm to 5 µm and later to 3.5 and 3 µm. Recently, manufacturers have been able to produce fully porous sub-2-µm silica particles or core–shell particles and columns packed with these materials achieved impressive separation efficiencies above 200,000 N/m (15–18).
However, HPLC columns packed with sub-2-µm particles show some disadvantages. The resulting column back pressure of these columns is above 400 bar (~6000 psi). Therefore, the effective use of such columns, especially with lengths in excess of 100-mm, may require dedicated ultrahigh-pressure chromatography (UHPLC) instrumentation, which permits operation at pressures over 1000 bar. In addition, the frictional heat generated during the operation of these columns at very high pressures might lead to problems in overall chromatographic performance. Furthermore, blockage of the column bed is often observed in columns packed with sub-2-µm particles because of small porosity frits (to contain these particles), as well as their smaller interstitial volumes. Unless column protection devices (for example, guard columns, in-line filters, or sample membrane filtration) are employed, column lifetime may be jeopardized.
The more recent success of core–shell materials with particle sizes of 2.5–3 µm is associated with the fact that they offer high separation efficiencies (as high as sub-2-µm particles) with a much more favorable column permeability and corresponding lower column back pressures (15,16). The improvement of separation efficiency is because of an improvement in all terms of the van Deemter equation:
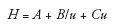
where H represents the theoretical plate height, A the eddy diffusion (great improvement here because of their narrow particle size distribution resulting in a more homogeneous packed bed), B the axial diffusion (which is inversely proportional to the linear velocity u), and C is the mass transfer depending on u.
The more favorable correlation of column back pressure and separation efficiency shown by core–shell particles as compared to fully porous particles also can be achieved with a new generation of silica-based monolithic HPLC columns (Chromolith HighResolution [HR]). Compared to the first generation of Chromolith columns, these new columns possess a more homogeneous porous silica network based on a well-designed silica skeleton in combination with a tailor-made bimodal pore structure (that is, macro- and mesopores).The chromatographic performance of this new generation of monolithic columns demonstrates improved separation efficiency and peak symmetry, especially for basic compounds, (for example, amitryptiline). In the following sections, the preparation and applications of these new columns is described.
Preparation of Silica-Based Monolithic HPLC Columns
Silica-based monolithic columns are prepared according to a sol-gel process utilizing tetramethoxysilane (TMOS) as a silica precursor and polyethylene oxide (PEO) as a pore-forming agent (19). The two components TMOS and PEO are dissolved under slightly acidic conditions and the resulting solution is filled into suitable gelation tubes and heated at moderate temperature (see Figure 1). These reaction conditions initiate the hydrolysis and polycondensation of the TMOS in the presence of the PEO. The following two processes then take place in parallel and compete with each other:
- transfer of the sol to the gel state forming the silica
- phase separation of the silica-rich phase and the solvent-rich phase.
The phase separation process that occurs after a specified reaction time determines the macroporous structure of the silica. In this stage, the silica-rich phase separates from the solvent-rich phase, building up the silica skeleton. Elimination of the solvent by drying in the solvent-rich phase then leads to the formation of the macropores. In Figure 1 the phase separation step is indicated by the appearance of the white rod in the gelation tube. Further polycondensation reactions cause a shrinkage of the rod, which then detaches from the inner surface of the gelation tube, allowing its removal.

Figure 1
The polycondensation of the TMOS starts along the PEO chains forming H-bonds to the polymer and building up the silica consecutively. During this reaction, methanol is introduced, which accumulates in "vacuoles" within the co-continuous silica-forming network. These "vacuoles" form the resulting macropores. Their size is determined by the concentration of the PEO added to the starting solution. An increasing amount of PEO will lead to silica monoliths with decreasing macropore sizes (20). The mesopore formation takes place at a later stage by thermal treatment. Because the mesopore size does not have a strong impact on the separation efficiency of the resulting columns, it will not be further discussed in this article.
The big difference between the first and new generations of silica-based monoliths is the macropore size, which in the case of the first generation (Chromolith columns) is around 2 µm and for the new generation (Chromolith HR columns) is around 1.1 µm.
Comparison of Silica-Based Monolithic HPLC Columns First Generation vs. Second Generation
The first generation of Chromolith columns has been commercially available for more than 10 years. Their preparation and chromatographic properties have been described in several papers (1–3). Table I shows physical and chemical data characterizing these columns. Chromolith columns of the first generation show performance similar to a 3.5–5 µm particle-packed column with a column back pressure similar to an 11-µm particle-packed column (21). These first generation columns can be coupled in series to add separation efficiency because of their high permeability. Figure 2 shows the separation of a mixture of six alkylbenzenes on 14 columns (100 mm × 4.6 mm each) coupled together for a total length of 1.4 m. The last peak gave 108,000 theoretical plates and corresponds to pentylbenzene, with a retention time of about 55 min. The mobile phase consisted of 80:20 (v/v) acetonitrile–water, and the corresponding column back pressure was only 117 bar (~1700 psi). This application clearly demonstrates the favorable permeability of the first-generation silica-based monolithic columns.
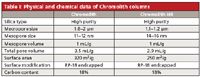
Table I
Furthermore, the rigid column bed of Chromolith columns with their macroporous through-pores of about 2 µm in diameter permits the direct analysis of "dirty samples" without prior sample preparation. Figure 3 shows the consecutive chromatography of six drugs (tetracyclines) dissolved in 10% albumin solution. In this case, 300 injections were done without serious loss of performance (N/m vs. number of injections). Consequently, Chromolith columns of the first generation show very long column lifetimes.
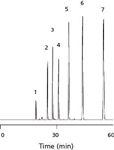
Figure 2
Chromolith HR columns possess much higher separation efficiencies than columns of the first generation (N/m > 140,000 vs. N/m > 80,000). During development of the second-generation monoliths, the focus has been on separation efficiency and peak symmetry. This has been achieved by systematically decreasing the macropore size and corresponding domain size. At the same time, a much more homogeneous porous silica network has been obtained, which causes a decrease in the eddy diffusion, or A term, of the van Deemter equation (equation 1). This results in much higher separation efficiencies. However, the separation efficiency increase has been achieved at the cost of a higher column backpressure because of the decrease of macropore size. While a 100 mm × 4.6 mm Chromolith column of the first generation can be operated at 25 bar (~370 psi) (60:40 [v/v] acetonitrile–water, 2 mL/min), an equivalent column of Chromolith HR shows a column back pressure of around 65 bar (~950 psi). Nevertheless, it has to be noted that typical lengths of Chromolith HR columns can still be employed with low pressure, conventional HPLC instrumentation.

Figure 3
Development and Applications of Chromolith HR Columns
Since their commercial availability in 2000, silica-based monoliths have been extensively studied by the scientific community regarding their performance in comparison to particle-packed columns. Among others, Guiochon and coworkers concluded that the first generation of monoliths shows radial heterogeneity, which prevents these columns from reaching separation efficiencies as high as that of comparable columns packed with <3-µm particles (25). This radial heterogeneity is inherently related to the sol-gel process, which is driven by a phase separation step within a gelation tube (as described above). A better control of this step in combination with a smaller domain size (equals the sum of macropore and skeleton diameter) and macropore structure has led to the second generation of monoliths, which show improved separation efficiency and peak symmetries. Monoliths of the second generation were prepared by systematically reducing the macropore size. Table II shows data of monoliths with a macropore size from 1.7 µm down to 1.0 µm, as well as their corresponding performance data (N/m and theoretical plate height H). The mesopore size of these monoliths was kept constant — around 15–16 nm (150–160 Å). From the data, it can be clearly seen that the separation efficiency of these columns is inversely proportional to the decrease in the macropore size. Furthermore, several batches of silica-based monoliths have been prepared with a constant macropore size of around 1 µm and variations in the mesopore size of about 12 –17 nm (120–170 Å). The corresponding data on the pore sizes and chromatographic performance are listed in Table III. It turns out that the average theoretical plate height for all of these columns is around H = 5.9 µm. It seems that the mesopore size has little impact on the separation efficiency of the Chromolith HR columns.

Table II
Recently, Tallarek and colleagues (22,23) were able to show that the porous silica structure of the Chromolith HR columns is much more homogeneous than that of the first generation. For this purpose, confocal laser scanning microscopy has been used as a tool to create images of the porous silica structure. A subsequent determination of so-called chord length distributions describing the variations of the macropore size and silica skeleton diameter reveals in a quantitative manner that Chromolith HR columns are much improved regarding homogeneity, which is in turn one reason for the higher separation efficiency (22). The other reason is a smaller macropore size, which together with the more homogenous structure has a dramatic effect on the eddy diffusion resulting in smaller A terms of the van Deemter equation and leads to smaller theoretical plate heights H.

Table III
The practical relevance of the structural improvements of Chromolith HR can be seen in Figures 4–6. Figure 4 shows the reversed-phase chromatographic separation of four tocopherols on a 3-µm porous particle–packed column (Figure 4a) and on the new monolithic column (Figure 4b) of the same dimensions and isocratic conditions. The peak symmetry of these components is slightly better on the Purospher STAR column, while separation efficiency and time of analysis is more favorable on the Chromolith HR column. Moreover, the baseline separation of a complex mixture of 32 pesticides under gradient conditions shown in Figure 5 demonstrates the "high resolution" power of these new generation monoliths. Finally, Figure 6 shows the separation of two antidepressants, imipramine and amitryptiline, with improved peak symmetries and subsequently low Tf (United States Pharmacopeia [USP]) values.
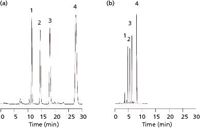
Figure 4
Summary and Future Perspective
Silica-based monoliths prepared by a sol-gel process can be improved on further regarding separation efficiency and peak symmetry (24–26). A decrease in the macropore size to 1.1 µm along with a decrease in the corresponding domain sizes leads to columns with a performance close to those of sub-2-µm and core–shell particle–packed columns (24). Furthermore, "down scaling" of the micro-porous silica structure was accompanied with a more radial homogeneity (22,24), which proved to reduce the eddy diffusion A term of the van Deemter equation, leading to higher efficiencies. In contrast to the columns packed with small particles, the monolithic Chromolith HR columns can still be operated with "low-pressure" conventional HPLC systems because of a very favorable column back pressure. This is at least two times lower than that of an equivalent particle-packed column. Despite these improvements, silica-based monoliths of the first generation are still needed. Because of their big macropores and their rigid column bed these columns are considered very robust materials that provide much longer lifetimes (by a factor of two) compared to conventional particle-packed columns. Furthermore, these columns are especially suitable for the direct analysis of "dirty samples" for which macropores of around 2 µm are quite effective in avoiding blockage of the column bed.

Figure 5
Silica-based monoliths have clearly improved since their commercial introduction 10 years ago. It is anticipated that the present structural features of the silica-based monoliths will be further improved. For this purpose, the sol-gel process being used for the preparation of the monoliths should be modified so that the domain size will be reduced further to a corresponding macropore size of less than 1 µm. The challenge will also be to control the inherent phase separation step in such a manner that it will lead to an even better homogeneity of the porous silica structure. It is not a dream any more to reach >200,000 N/m with a silica-based monolithic column; it is a reality. To reach efficiencies above 350,000 N/m is, in the opinion of this author, only a matter of time.
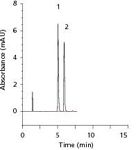
Figure 6
Acknowledgment
The author would like to thank the Chromolith team with members from sales and marketing, production, and R&D for their continuous engagement. Special thanks to Dieter Tanzer and David Lentz for their encouragement and many valuable discussions.
Karin Cabrera is head of R&D Analytical Chromatography at Merck Millipore, Merck KGaA, in Darmstadt, Germany.
Direct correspondence to: karin.cabrera@merckgroup.com.
References
(1) G. Guiochon, J. Chromatogr. A 1168, 101–168 (2007).
(2) K. Cabrera, J. Sep. Sci. 27, 843–852 (2004).
(3) Monolithic Silicas in Separation Science, K.K. Unger, N. Tanaka, and E. Machtejevas, Eds. (Wiley-VCH Verlag GMBH, Weinheim, Germany, 2011).
(4) H. Kobayashi, T. Ikegami, H. Kimura, T. Hara, D. Tokuda, and N. Tanaka, Anal. Sci. 22(4), 491–501 (2006).
(5) S. El Deeb, Chromatographia, 74(9–10), 681–691 (2011).
(6) G. Ashraf and T. Ikegami, J. Sep. Sci. 34(16–17), 1945–1957 (2011).
(7) F. Svec and C. Huber, Anal. Chem. 78(7), 2100–2108 (2006).
(8) F. Svec, J. Sep. Sci. 27(17–18), 1419–1430 (2004).
(9) F. Svec and J.M.J. Frechet, in Monolithic Materials: Preparation, Properties and Applications, F. Svec, T.B. Tennikova, and Z. Deyl, Eds. (Elsevier, Amsterdam, 2003).
(10) F. Svec, J. Sep. Sci. 32(1), 3–9 (2009).
(11) F. Svec, J. Chromatogr. A 1217(6), 902–924 (2010).
(12) J.O. Omamogho, E. Nesterenko, D. Connolly, and J.D. Glennon, LCGC No. America 30(S4), 54–58 (2012).
(13) J.J. DeStefano, T.J. Langlois, and J.J. Kirkland, J. Chromatogr. Sci. 46(3) 254–260 (2008).
(14) D. Guillarme, J. Ruta, S. Rudaz, and J.-L. Veuthey, Anal. Bioanal. Chem. 397(3), 1069–1089 (2010).
(15) F. Gritti and G. Guiochon, J. Chromatogr. A 1157, 289–303 (2007).
(16) F. Gritti, I. Leonardis, D. Shock, P. Stevenson, A. Shalliker, and G. Guiochon, J. Chromatogr. A 1217, 1589–1603 (2010).
(17) U. Neue, D. Diehl, and P. Iraneta, presented at the Pittcon Conference & Expo, Chicago, Illinois, 2009.
(18) F. Gritti and G. Guiochon, J. Chromatogr. A 1216, 1353–1362 (2009).
(19) K. Nakanishi, J. Por. Mat. 4, 67–112 (1997).
(20) S. Altmaier and K. Cabrera, J. Sep. Sci. 31, 2551–2559 (2008).
(21) F.C. Leinweber and U. Tallarek, J. Chromatogr. A 1006, 207–228 (2003).
(22) K. Hormann, T. Muellner, S. Bruns, A. Hoeltzel, and U. Tallarek, J. Chromatogr. A 1222, 46–58 (2012).
(23) S. Bruns, T. Müllner, M. Kollmann, J. Schachtner, A. Hoeltzel, and U. Tallarek, Anal Chem. 82(15), 6569–6575 (2010).
(24) F. Gritti and G. Guiochon, J. Chromatogr. A 1225, 79–90 (2012).
(25) F. Gritti and G. Guiochon, J. Chromatogr. A 1218, 5216–5227 (2011).
(26) F. Gritti and G. Guiochon, J. Chromatogr. A 1216, 4752–4767 (2009).

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).














