Next-Generation Stationary Phases: Properties and Performance of Core-Shell Columns
Special Issues
A brief review of the physicochemical properties of core–shell particles and their chromatographic efficiencies compared with those of sub-2-µm fully porous particles
This short review focuses on the physicochemical properties of core–shell particles and their resulting chromatographic efficiencies as they relate to the comparison of their mass transfer kinetics to those of columns packed with sub-2-µm fully porous particles.
In recent years, column technologies have diversified for a multitude of separation applications. The improvement in separation efficiency attainable today on modern liquid chromatography (LC) columns results from the evolution of new packing materials and sorbents that offer analysts the choice to use fully porous particles, core–shell particles, or silica–polymer monolithic columns, depending on the application (1). Among the new column packing materials, core–shell particles, also called superficially porous particles, of sub-3-µm diameter have gained considerable attention because of significant improvements in separation efficiency. Columns packed with core–shell particles can generate a minimum plate height below H ~ 3.5 (2,3) for small molecules, a similar efficiency to that of columns packed with fully porous sub-2-µm particles.
The use of core–shell particles as column packing materials is not new; in fact, they have been in existence and have been used since the early days of high performance LC (HPLC) (4–6). The main purpose of pellicular particles in the early days of HPLC was to improve the mass transfer resistance of large molecules to facilitate faster separation (6). However, pellicular particles later met their demise because such particles were not as robust and refined as the fully porous particles that were introduced later. For detailed historical accounts of the development of various pellicular particles as packing materials for HPLC columns, readers should refer to reference 7. The new-generation core–shell particles display significant improvements in important physicochemical parameters, such as particle-size distribution; thickness of the porous shell, providing substantial increase in surface area for retention (8); surface roughness or smoothness; and the purity and robustness of the base core–shell silica. A number of current manufacturers and academic researchers currently are investing a great deal of time in the preparation of core–shell silica particles (9); a list appears in Table II in the Majors article in this issue (10). The new-generation core–shell particles have been solely designed to compete with the sub-2-µm fully porous particles by providing a substantial increase in column permeability; this results in faster separations while maintaining the same high column efficiencies. Recently, another set of core–shell particles (Phenomenex's Aeris core–shell silica particle) has been released and is claimed to be a rival to the sub-2-µm fully porous particles for separating large molecules (11). This short review will summarize the key physiochemical properties of the new generation of core–shell particles that results in superior performance of HPLC columns.
Physical Properties of Core–Shell Particles
Shell Thickness
In contrast to earlier core–shell packing materials, the volume fraction of the porous permeable layers around the solid impermeable core of the new generation of core–shell particles (which are generally 2.6, 2.7, or 1.7 µm in diameter) is 29–37%, as opposed to <10% for the earlier core–shell particles (5,12). For the separation of small molecules, increasing the shell thickness is of paramount importance because it contributes to solute retentivity and minimizes the A-term contribution to band broadening (13). For example, the 2.7- and 2.6-µm commercially available core–shell particles provide an excellent peak capacity under gradient elution with holdup time as low as 10 s, which requires an inlet pressure of 400 bar (14,15).
Figure 1 shows electron micrographs of how porous shell thickness is controlled while keeping the overall core–shell particle diameter constant. Gritti and Guiochon (16) have examined in detail the improvement in column separation efficiency brought by the new core–shell particles as a result of their thicker porous shell. Modern-day core–shell particles now have larger surface areas (~100–150 m2 /g) compared to their predecessors (< 1–13 m2 /g), which results in significant gains in sample loading capacity and enhanced stability of the stationary phase.

Figure 1
Surface Roughness
Literature reports have shown that the surface roughness of core–shell particles plays a major role in enhanced film mass transfer resistance, an independent contribution to band broadening caused by the rugosity of the particle surface (17). A recent study by Gritti and Guiochon has shown that core–shell particles with large pore sizes can alleviate the film mass transfer resistance (18).
Figure 2 shows the external surface structure of two sub-3-µm core–shell particles: prototype 2.6-µm core–shell particles synthesized in the ISSC Cork laboratory (Figure 2a); Kinetex 2.6-µm core–shell particles (Phenomenex) (Figure 2b); and 3.5-µm fully porous particles (Figure 2c). The prototype material has a similar smooth surface in comparison to the fully porous particles. Conversely, the Kinetex 2.6-µm material is unique with its rough surface. The impact of the surface roughness on the packed bed porosity heterogeneity is still a matter of debate.

Figure 2
Particle-Size Distribution and Column Packing
The degree of the packed-bed radial heterogeneity of the external porosity across the column is directly proportional to the A term of the van Deemter equation. Traditionally, there is a general consensus that a narrow particle-size distribution is the major source of improvement of the A term of columns packed with core–shell particles (19,20), it was recently reported that subtle changes in the column packing methodology itself can greatly improve packed beds made of particles with a broader size distribution (21), provided fines of particles of the same fractional size of the external porosity are eliminated. Using a numerical packing algorithm, Tallarek and colleagues (22) studied the relationship of particle-size distribution in unconfined packing (without a wall) generated over a range of bed porosities (external porosities) to flow permeability and the A term of the van Deemter equation. The A term is expected to be more sensitive to bed porosity heterogeneity than to particle-size distribution. An example of the relationship of particle-size distribution and efficiency was demonstrated at the ISSC Cork laboratory. As shown in Figure 3, two batches of prototype 2.6-µm core–shell particles were characterized and differ significantly in particle size distribution (Figure 3a and 3b). These different packing materials were identically packed in 100 mm × 2.1 mm columns and their corresponding height equivalent to a theoretical plate (HETP) plot is given in Figure 3c, which shows no discernible differences. This result implies that particle-size distribution has a minimal effect on overall column efficiency.

Figure 3
Core–Shell Particle Chromatographic Efficiency
Height Equivalent to a Theoretical Plate
Columns packed with 2.7- and 2.6-µm core–shell particles consistently generate reduced plates heights of 1.5 and 1.3, respectively, in 4.6 mm i.d. column formats, generally outperforming columns packed with fully porous particles (h > 2.0) in the same column internal diameter under identical conditions. This improvement in core–shell column efficiency is attributed to the mechanism of the mass transfer kinetics, which ultimately plays a pivotal role in the contributions to the HETP. The HETP is a combination of eddy and longitudinal diffusion and resistance to solute mass transfer in the stationary phase present inside the pores and outside the particle surface (the latter is referred to as film mass transfer resistance) and is represented as
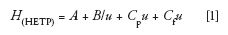
where A and B are the coefficients of the eddy and longitudinal diffusion, respectively, Cpu and Cfu are the trans-particle and film mass transfer resistance coefficients, respectively. For detailed description of the physicochemical aspects of the different mass transfer coefficients and their contributions to HETP, readers are referred to reference 23.
Eddy Diffusion
Conventional wisdom generally accepts that the A term of solute mass transfer is unaffected by flow velocity of the mobile phase and that the particle-size distribution is the determinant factor of the A term. The evolution of modern core–shell particles appears to confirm a close relationship between particle-size distribution and the A term. However, recent studies on packing reconstruction using computer simulation have elucidated the structure–transport relationship of packed beds and demonstrated that the current state of particle-size distribution commonly found in commercial packings of fully porous particles had only a minor effect on the A term (24,25). The relationship between the slurry packing methodology and the randomness of interparticle pore spaces present in the packed bed from the column center to the wall is now becoming an avenue to explore (26), because this is the true parameter that influences eddy diffusion. Because the currently existing commercial sub-3-µm core–shell particles seem to generate bed porosity that mimics loose packing and can still generate very high efficiency, it can be said that the bed porosity is far less heterogeneous from the center of the column to the wall in comparison to columns packed with fully porous particles. Gritti and Guiochon have demonstrated that there is a strong link between retention and the A term for small molecules at ko = 0 to ko = 3 (16,27).
Figure 4 illustrates the relationship between retention factors of different solutes and the reduced A term for columns packed with fully porous particles and core–shell packing (16). The A term for nonretained solute (uracil) is far superior for the core–shell column than for the fully porous column at high flow rates. The improvement of the A term becomes more significant for well-retained solutes as a result of the impact of retention on the A term, which ultimately is larger for fully porous particles.

Figure 4
Longitudinal Diffusion
The B term is related to the bulk molecular diffusivity, Dm, according to the following equation:
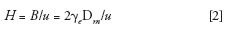
where γe is the obstruction factor. The B term in the HETP equation is systematically reduced in core–shell particles compared to the fully porous packing. Given that solute diffusion in the stationary phase is bound to be slower than that in the bulk eluent, the kinetics of diffusion are enhanced at the interface of the solute and stationary phase and results in additional mass transfer effects referred to as surface diffusion (28), which increases with stationary-phase surface area. Surface diffusion is minimal in core–shell particles by virtue of the presence of the impermeable solid core particles; thus the B term is smaller for the core–shell particles and at zero to very low flow rates. The B term can be measured experimentally by the peak parking method (3) and the same method (after subtle modification) also has been reported to estimate the bulk molecular diffusivity, Dm (29).
Trans-Particle and Film Mass Transfer Resistance
The improvement in trans-particle mass transfer resistance (the C term) in columns packed with core–shell particles is not significantly greater than it is with the fully porous particles for the separation of small molecules. This is because of the larger contribution of surface diffusion (28) found in fully porous particles (which have a larger specific surface area in comparison to core–shell particles of the same pore size), which increases the particle diffusivity, Dp (a contribution of both pore and surface diffusion) for molecules with faster Dm. With large molecules, however, the pore size of the core–shell particles greatly improves Dp (18). This can afford an improved C term for large molecules. The Aeris WidePore core–shell particles serve this purpose, allowing even faster separation of macro-molecule and proteins without loss of efficiency (30).
The film mass transfer resistance coefficient is larger for the new generation of core–shell particles, especially for large molecules, as a result of the particles' surface roughness, a property that fully porous particles, with their smooth surfaces, lack (17). A substantial increase in pore size has been reported to alleviate the film mass transfer resistance (18); this is the advantage of the Aeris core–shell particles that are designed with large pores.
Frictional Heat Effects
The frictional heat effect of columns packed with core–shell particles is only severe with sub-2-µm particles. However, in comparison to the fully porous sub-2-µm particles, the solid core present in the core–shell particles promotes better heat conductivity and frictional heating become preponderant on the fully porous sub-2-µm particles, especially when operated at high linear velocity (31). The low permeability of columns packed with sub-2-µm particles inherently causes large heat generation that changes the properties of the mobile phase that carries the solute through the columns. The sub-3-µm core–shell particles are now the solution to heat effect when very fast analysis is desired.
Concluding Remarks
A brief summary of the relationship of the physicochemical and mass transfer kinetic properties of the recently evolved core–shell particles has been given, based on recent scientific reports. The superior performance of sub-3-µm core–shell particles is largely attributed to the significant improvement in their manufacture, resulting in improvement in their physicochemical properties compared to the early day core–shell particles. The advantage of the lower permeability brought by the sub-3-µm core–shell particles greatly surpasses those of the sub-2-µm fully porous particles to allow a significant increase in speed of analysis. The future of core–shell particles will see further dramatic changes in porous shell design and configuration, including possible decreases in particle diameter to increase the speed of analysis even further (11).
Acknowledgment
J.O.O. would like to thank Elaine Stack, a PhD student working in the ISSC Cork laboratory, for producing the HETP data shown in Figure 3e. Special thanks to Science Foundation Ireland for the financial support (Grant Number 08/SRC/B412) for research funding of the Irish Separation Science Cluster (ISSC) under the Strategic Research Cluster Programme.
Ekaterina Nesterenko and Damian Connolly are with the Irish Separation Science Cluster (ISSC) at Dublin City University in Glasnevin, Dublin, Ireland.
Jesse O. Omamogho and Jeremy D. Glennon are with the Irish Separation Science Cluster (ISSC) and Analytical and Biological Chemistry Research Facility (ABCRF) in the Department of Chemistry at the University College Cork, in Cork. Ireland. Please direct correspondence to: j.glennon@ucc.ie and j.omamogho@ucc.ie. ?
References
(1) K.K. Unger, R. Skudas, and M.M. Schulte, J. Chromatogr. A 1184, 393 (2008).
(2) F. Gritti, I. Leonardis, D. Shock, P. Stevenson, A. Shalliker, and G. Guiochon, J. Chromatogr. A 1217, 1589 (2010).
(3) F. Gritti, I. Leonardis, J. Abia, and G. Guiochon, J. Chromatogr. A 1217, 3819 (2010).
(4) J.J. Kirkland, Anal. Chem. 37, 1458 (1965).
(5) J.J. Kirkland, Anal. Chem. 41, 218 (1969).
(6) C.G. Horvath, B.A. Preiss, and S.R. Lipsky, Anal. Chem. 39, 1422 (1967).
(7) G. Guiochon and F. Gritti, J. Chromatogr. A 1218, 1915 (2011).
(8) J.O. Omamogho, J.P. Hanrahan, J. Tobin, and J.D. Glennon, J. Chromatogr. A 1218, 1942 (2010).
(9) S. Fekete, E. Olah, and J. Fekete, J. Chromatogr. A 1228C, 57 (2012).
(10) R.E. Majors, LCGC No. America 30(S4), 8–19 (2012).
(11) http://www.phenomenex.com/webdocument/aeris_bro.pdf.
(12) J.J. Kirkland, Anal. Chem. 64, 1239 (1992).
(13) F. Gritti and G. Guiochon, AIChE J. 56, 1495 (2010).
(14) S. Fekete, J. Fekete, and K. Ganzler, J. Pharm. Biomed. Analysis 49, 64 (2009).
(15) E. Oláh, S. Fekete, J. Fekete, and K. Ganzler, J. Chromatogr. A 1217, 3642 (2010).
(16) F. Gritti and G. Guiochon, J. Chromatogr. A 1217, 8167 (2010).
(17) F. Gritti and G. Guiochon, J. Chromatogr. A 1166, 30 (2007).
(18) F. Gritti and G. Guiochon, J. Chromatogr. A 1218, 907 (2011).
(19) D. Cabooter, A. Fanigliulo, G. Bellazzi, B. Allieri, A. Rottigni, and G. Desmet, J. Chromatogr. A 1217, 7074 (2010).
(20) A. Liekens, J. Billen, R. Sherant, H. Ritchie, J. Denayer, and G. Desmet, J. Chromatogr. A 1218, 6654 (2011).
(21) F. Gritti, T. Farkas, J. Heng, and G. Guiochon, J. Chromatogr. A 1218, 8209 (2011).
(22) A. Daneyko, A. Höltzel, S. Khirevich, and U. Tallarek, Anal. Chem. 83, 3903 (2011).
(23) F. Gritti and G. Guiochon, J. Chromatogr. A 1221, 2 (2012).
(24) S. Khirevich, A. Daneyko, A. Höltzel, A. Seidel-Morgenstern, and U. Tallarek, J. Chromatogr. A 1217, 4713 (2010).
(25) S. Khirevich, A. Höltzel, A. Daneyko, A. Seidel-Morgenstern, and U. Tallarek, J. Chromatogr. A 1218, 6489 (2011).
(26) S. Bruns and U. Tallarek, J. Chromatogr. A 1218 (2011) 1849.
(27) F. Gritti and G. Guiochon, J. Chromatogr. A 1217, 6350 (2010).
(28) K. Miyabe and G. Guiochon, J. Chromatogr. A 1217, 1713 (2010).
(29) K. Miyabe, N. Ando, and G. Guiochon, J. Chromatogr. A 1216, 4377 (2009).
(30) S. Fekete, R. Berky, J. Fekete, J-L. Veuthey, D. Guillarme, J. Chromatogr. A, doi:10.1016/j.chroma.2012.03.018.
(31) F. Gritti and G. Guiochon, J. Chromatogr. A 1217, 5069 (2010).

Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












