Surfing on Mobile Phase, Part I: Origins of Mobile-Phase Composition Waves and Their Effects on Detector Baselines
Liquid chromatography (LC) pumps produce mobile-phase streams with small short-term variations in mobile phase composition. We explain the origin of these variations and their effects on chromatographic performance.
The most commonly used designs for modern liquid chromatography (LC) pumps produce mobile-phase streams with small short-term variations in mobile phase composition. Understanding the origin of these variations and their effects on chromatographic performance can help us develop high-performing methods. In this installment, we focus on the effect of these mobile-phase composition “waves” on detector baselines.
This month’s installment of “LC Troubleshooting” is motivated by communication I had with readers in recent months about mobile-phase composition, pumps, and mixing. Although these topics have been discussed in this column frequently in the past, they are moving targets to some extent because pump technology for liquid chromatography (LC) continues to evolve. Therefore, user expectations for pump performance in demanding applications also change. As an example of the ongoing importance of these issues, below is an excerpt of an e-mail I received recently from an LC user:
“We have two [brand name LC instruments] with quaternary pumps that are acting up. If we try to run the system using 50% A and 50% B, we get retention time shifts. If we pre-mix the solvent and just run channel A, all is fine. Gradients make the problem worse...so obviously there is a problem with the mixing.”
In this installment, I review the operating principles of LC pumps that rely on low- or high-pressure mixing approaches, describe how waves of solvent composition can develop in the mobile phase, and evaluate the potential impacts of these waves on detector baselines. In next month’s installment, I will discuss the results of simulations that show how these waves can impact variability in retention time along with some solutions to these problems. One could devote an entire book chapter to these topics, so I discuss the highlights here, providing a concise overview of LC pump technology. For readers that are interested in learning more, I encourage considering the following resources: the books by Kromidas (1) and Snyder and Dolan (2) have chapters dedicated to modern LC pump technology, details about performance specifications, and descriptions of tests that can be used to evaluate pump performance; two “LC Troubleshooting” articles by John Dolan in 2006 (3) and 2014 (4) describe case studies that illustrate what can happen when things go wrong in the pump; and Choikhet and co-workers (5) and Gritti (6) discuss in great detail the impact of imperfect pump performance on LC applications involving trifluoroacetic acid (TFA) in the mobile phase. These are all excellent resources for those looking to add to their LC troubleshooting knowledge.
Review of LC Pumping Principles—Low- and High-Pressure Mixing Designs
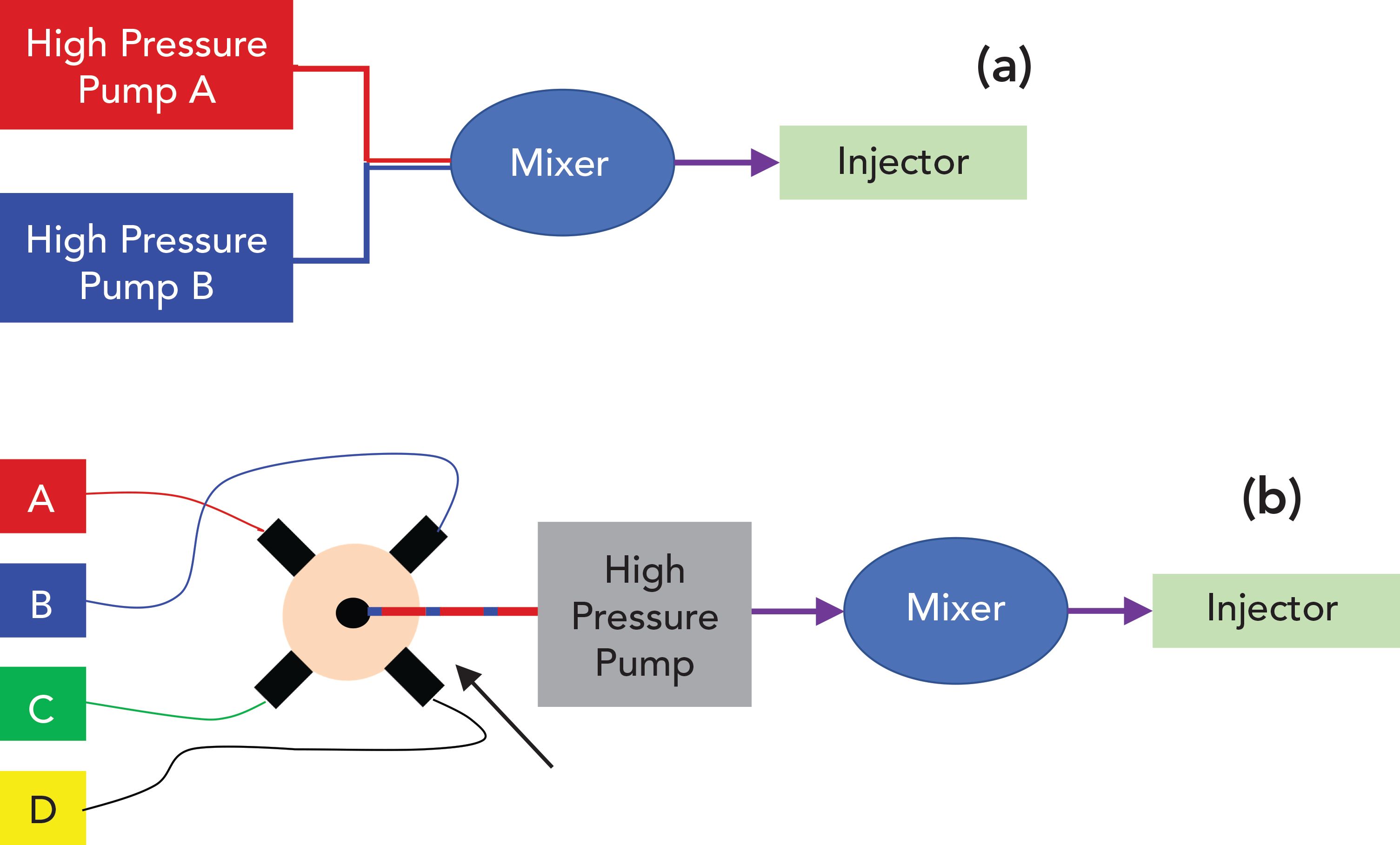
Figure 1 illustrates the basic principles of the two of the most common designs for pumping systems used in LC. In the case of the high-pressure mixing approach, two independent pump heads, each capable of producing a high-pressure stream of a mobile-phase component, draw in and discharge solvent at a consistent flow rate (for isocratic operation). For example, if the total flow rate through the column is 1.0 mL/min, and the desired mobile-phase composition is 40:60 acetonitrile:water, then one pump head discharges acetonitrile continuously at 0.4 mL/ min, and the other pump head discharges water continuously at 0.6 mL/min. The two streams discharged from the pump heads then converge and pass through a mixer, and the mixed mobile phase proceeds to the sampler, and eventually the column. In the case of the low-pressure mixing approach, there is only one pump head that pressurizes the mobile phase to drive it through the column. The mobile-phase composition is determined by assembling small “packets” of individual solvents in a serial fashion into a mobile-phase stream that is drawn into the high-pressure pump. In most modern pumps of this design, the volume of each solvent packet is determined by the length of time a solenoid-type valve is open between the solvent bottle and the proportioning valve unit. Furthermore, these times are also related to the volume of each stroke of the high-pressure pump and the mobile-phase flow rate. For example, suppose the stroke volume is 100 μL, the desired mobile-phase composition is 40:60 acetonitrile:water, and the flow rate is 1 mL/min. The period of each pump stroke will be 6 s; the solenoid for the acetonitrile line will be open for 2.4 s, drawing pure acetonitrile into the tubing leading from the proportioning valve to the pump head. Then, this valve closes, and the solenoid for the water line opens for 3.6 s and pure water is drawn into the tubing. This completes one cycle of mobile-phase composition proportioning. The solvent composed in this way is mixed extensively as it travels through the high-pressure pump head itself, and an additional mixer is positioned between the pump and the sampler.
FIGURE 1: Block diagrams for the two most commonly used designs of high performance liquid chromatography (HPLC) pumps in use today: (a) binary pump with high-pressure mixing; and (b) quaternary pump with low-pressure mixing.
Origins of Solvent Waves and Their Impacts on Detector Baselines
Each of the pump designs discussed above has several strengths and weaknesses. In both cases, however, the mobile-phase composition at the pump outlet will not be perfectly smooth (that is, no variation in composition during isocratic operation; in gradient elution, the change in composition over time would ideally be smooth without any short-term noise). The primary causes of the deviations of the actual mobile-phase composition from what is programmed are different for the two designs. In the case of the low-pressure mixing approach, it is intuitive that there would be short-term variations in composition on the time scale of one pump stroke. During the pump stroke, there are times when the fluid entering the high-pressure pump is literally all A or all B. This is illustrated in Figure 2b. The resulting variation in mobile-phase composition can be smoothed to a large degree with effective mixing downstream from the high-pressure pump, but completely eliminating the variation would require a large volume mixer. If the detector is capable of detecting small variations in composition (for example, refractive index detection, or ultraviolet [UV] detection in the case where mobile-phase additives absorb UV light, such as TFA), then they will be observable in the detector signal as “waves,” or short-term noise. Adding a large mixer introduces other problems, such as a large delay between the time of a programmed change in composition and when that change arrives at the column (in gradient elution this appears as the “gradient delay” or “dwell” time). Thus, the configurations of these pumps as received from manufacturers reflect a compromise between doing enough mixing to smooth out these waves to a large extent and not adding a mixer that is so large that it causes other problems. Indeed, pump manufacturers offer mixers of different volumes that allows the user to choose a larger volume mixer for applications that are expected to be especially sensitive to short-term variations in mobile-phase composition (for example, applications involving TFA).
FIGURE 2: Conceptual illustration of the origin of mobile phase composition waves in the case of (a) high-pressure mixing and (b) low-pressure mixing designs used in modern pumps.
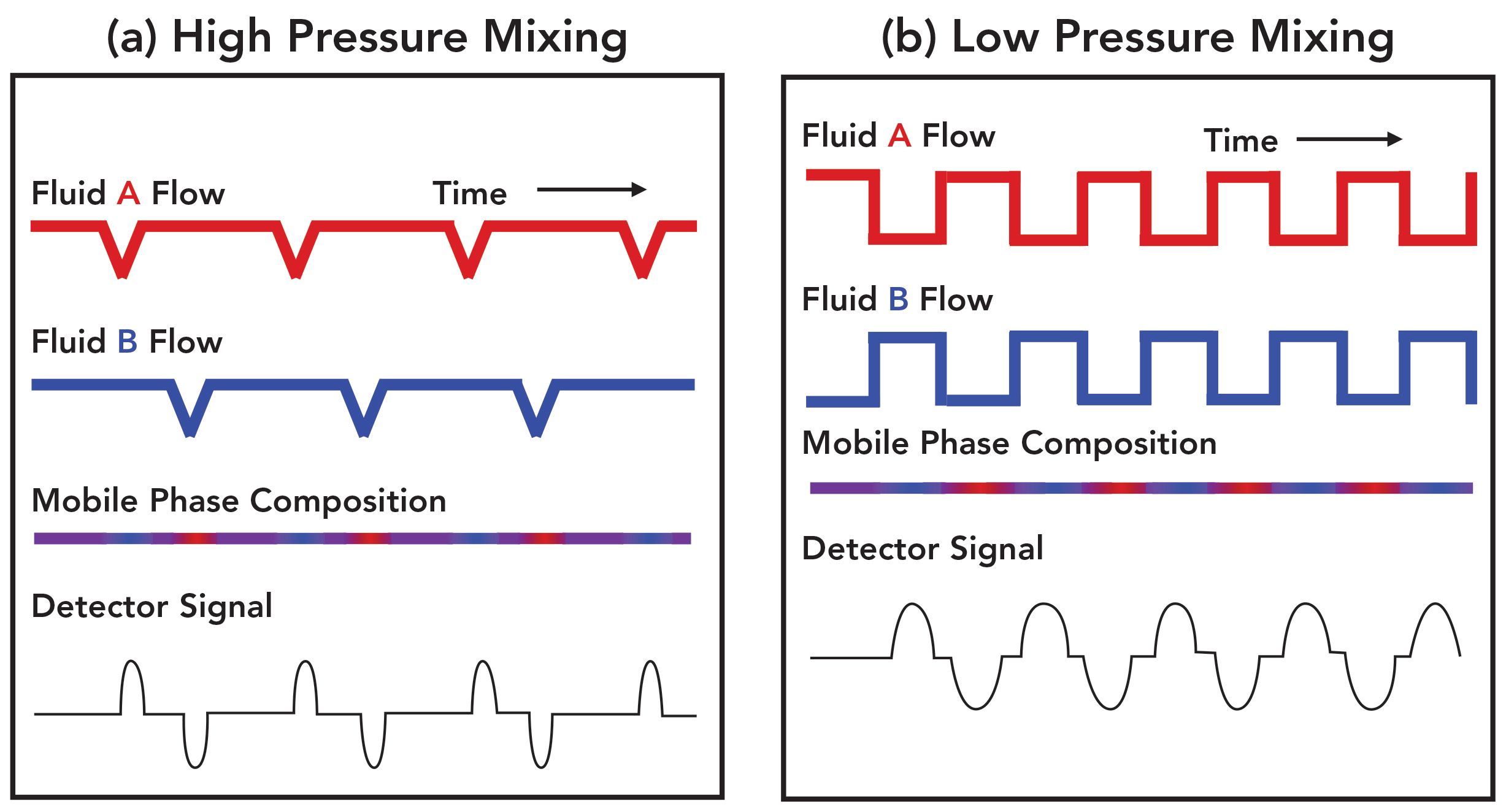
The primary origin of solvent waves in the case of high-pressure mixing is fundamentally different. In this case, if the flow rate from each pump head were perfectly consistent over time, the composition of the mixed mobile phase would be perfectly consistent over time. But, in a reciprocating piston design (which is the dominant design in use today), there are small changes in the flow from each pump head at the end of a piston stroke due to the imperfect operation of check valves. These small changes in flow are illustrated in Figure 2a. If these flow rate changes are different for channels A and B, and they are not perfectly synchronized in time, then there will be a small, short-term variation in the composition of the mixed mobile phase. The lengths of the resulting waves in this case tend to be shorter in comparison to the low-pressure mixing case, as illustrated in Figure 2. These waves can also be greatly minimized by introducing a mixer between the solvent convergence point in the pump and the LC column. However, the same challenge exists here as with the low-pressure mixing design: Completely eliminating the waves requires a large mixer, so what we use in practice represents a compromise between smoothing the mobile-phase composition and having a low gradient delay volume (which is essential for fast gradient elution separations, for example).
As mentioned above, the mobile-phase composition waves that flow from the pump can impact the quality of detector baselines (as measured by noise and drift) if the detector signal is dependent on the composition of the mobile phase itself. Most of the time, this can be avoided or minimized through judiciously choosing the conditions and instrument parameters. For example, acetonitrile is attractive as a mobile-phase organic solvent modifier for reversed-phase liquid chromatography (RPLC) when using UV detection at low wavelengths (< 230 nm). If the mobile- phase components are effectively transparent to the detector, then small variations like waves in the composition will not impact the quality of the detector baseline signal. Sometimes, though, this is impossible, or at least very difficult to avoid. One well known and studied example is the case where TFA is used as a mobile-phase modifier for RPLC separations of peptides involving UV detection. TFA is attractive because it tends to improve peak shapes for peptides and increase retention for hydrophilic peptides. However, a disadvantage of TFA in this context is that it absorbs a significant amount of UV light at 214 nm, which is the wavelength typically used for peptide mapping applications. Furthermore, the TFA itself is somewhat retained by RPLC stationary phases. When a mobile-phase composition wave travels through the column, the acetonitrile-rich part of the wave will decrease the local retention of TFA, dumping more of it into the mobile phase where it will absorb more UV light. This is a very complex situation that cannot be thoroughly discussed here, but there are at least two excellent papers (5,6) that describe all of the factors involved and demonstrate the impact of different chromatographic variables on baseline quality when using TFA in the mobile phase with UV detection. I strongly encourage readers interested in learning about this situation in more detail to consult these papers.
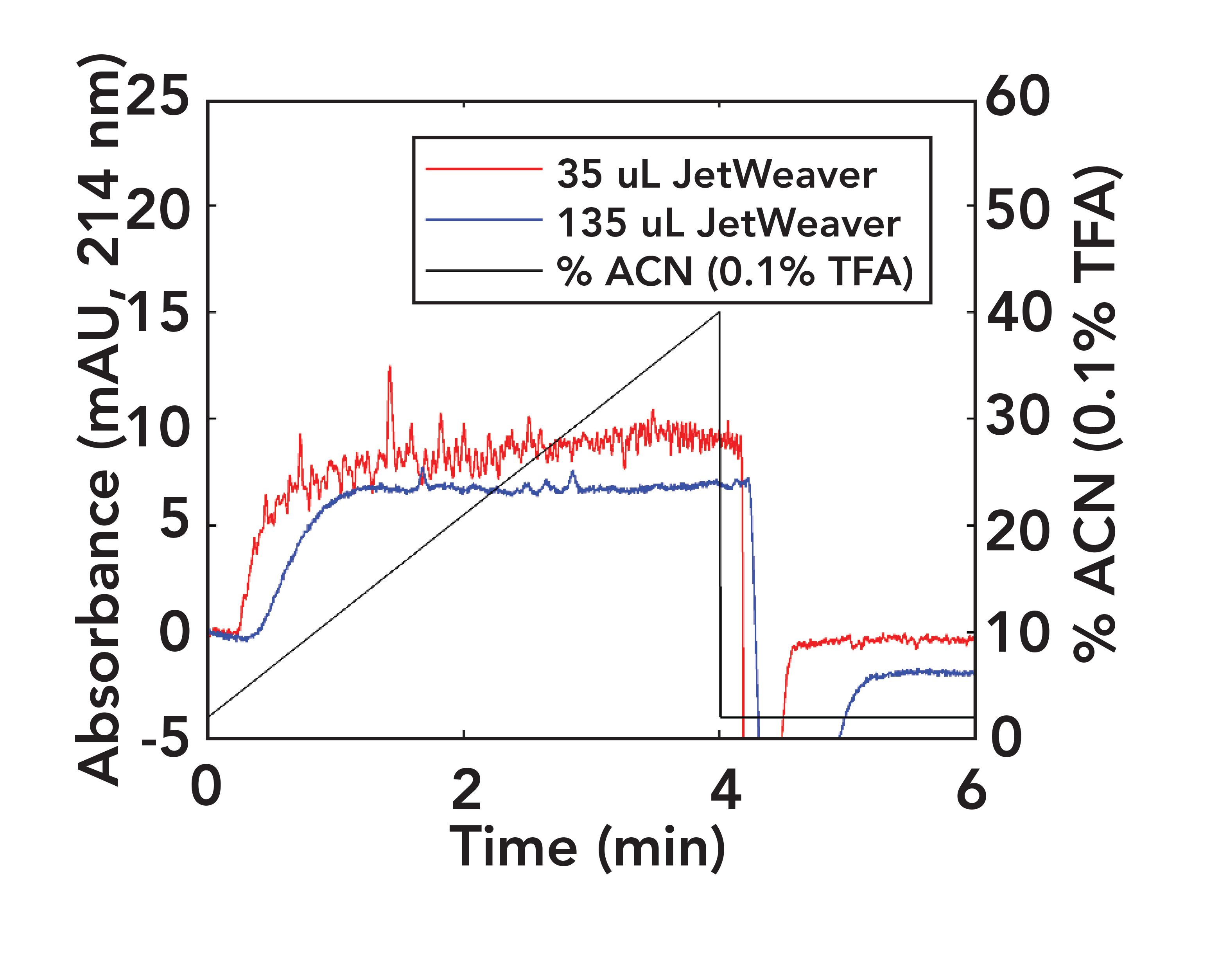
In a previous installment of “LC Troubleshooting”, I discussed why mobile-phase mixers are needed following LC pumps, and the types of situations when a change in the type of mixer might be needed (7). Figure 3 shows the effect of increased mixer volume on the noise level in UV detector baselines when using TFA in the mobile phase delivered by a binary pump. Although the 35 μL mixer might be adequate for other applications, a larger volume mixer is helpful for reducing baseline noise in this case because of the increased sensitivity of the baseline noise to mobile-phase composition waves when using TFA as an additive.
FIGURE 3: Comparison of ultraviolet (UV) absorbance signals (214 nm) obtained with different mixers in use. The pump was a high-pressure binary mixing system (Agilent 1290, Infinity II). The column was a 30 mm x 2.1 mm i.d. Agilent SB-C18, and gradient elution was used. Solvent A was 0.1% trifluoroacetic acid (TFA) in water, solvent B was 0.1% TFA in acetonitrile (ACN), and the gradient ran from 2–40% B in 4 min.
Summary
In this column, I have reviewed the operating principles of modern LC pumps based on low- and high-pressure mixing designs and explained how these pumps produce mobile-phase streams with small short-term variations in mobile-phase composition. These composition “waves” can negatively affect detector baseline quality and also retention time variability. In next month’s installment of “LC Troubleshooting”, I will continue exploring this topic by discussing the results of simulations that illustrate the effect of method parameters including the amplitude of the composition waves, flow rate, and pump stroke volume on retention time precision.
References
(1) S. Kromidas, Ed., The HPLC Expert II: Find and Optimize the Benefits of your HPLC/UHPLC (Wiley-VCH, Weinheim, Germany 2017).
(2) L.R. Snyder and J.W. Dolan, High-Performance Gradient Elution: The Practical Application of the Linear-Solvent-Strength Model (John Wiley & Sons, Hoboken, New Jersey, 2007).
(3) J.J. Gilroy and J.W. Dolan, LCGC North Am. 24, 662–667 (2006).
(4) J. Chow and J.W. Dolan, LCGC North Am. 30, 312–318 (2012).
(5) K. Choikhet, B. Glatz, and G. Rozing, LCGC Europe 16(2), 2–9 (2003).
(6) F. Gritti, J. Chromatogr. A. 1633, 461605 (2020). https://doi.org/10.1016/j. chroma.2020.461605.
(7) D. Stoll, LCGC North Am. 36, 746–751 (2018).
About The Column Editor

Dwight R. Stoll is the editor of “LC Troubleshooting.” Stoll is a professor and the co-chair of chemistry at Gustavus Adolphus College in St. Peter, Minnesota. His primary research focus is on the development of 2D-LC for both targeted and untargeted analyses. He has authored or coauthored more than 60 peer-reviewed publications and four book chapters in separation science and more than 100 conference presentations. He is also a member of LCGC’s editorial advisory board. Direct correspondence to: LCGCedit@mmhgroup.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








