Modern Trends in Mixed-Mode Liquid Chromatography (LC) Columns
Mixed-mode chromatography columns are on the rise. We review recent fundamental research, stationary phase development and design, and areas of application.
Commercialization of columns that provide multiple modes of chromatographic separations have recently been on the rise. For example, combinations of retention modes, such as ion-exchange and reversed-phase, often enable the separation of complex mixtures of analytes not possible using single-mode columns. In this work, recent trends in what is often referred to as “mixed-mode” phase are investigated. Particular attention is paid to recent fundamental research, stationary phase development and design, and areas of application.
In recent LCGC reviews of newly launched high-performance liquid chromatography (HPLC) columns, it was observed that several stationary phase developments fell into a category that can be characterized as “mixed-mode” (1,2). The idea of mixed-mode chromatography is not new. However, the observation prompted the question, “What is the current status of mixed-mode chromatography?”
An initial search through the literature and various websites revealed that there have been a multitude of publications, including reviews, that have written about mixed-mode chromatography in recent years. For example, West and others published a review of mixed-mode chromatography that covered many important developments, including combinations of reversed-phase liquid chromatography (RPLC) and ion-exchange chromatography (IEC), as well as hydrophilic interaction liquid chromatography (HILIC) and ion-exchange (3). Zhang and Liu published another notable review that focused on mixed-mode chromatography applications in pharmaceutical and biopharmaceutical analyses (4). The reader is referred to these reviews and references within for a more in-depth discussion of the technology.
Mixed-mode chromatography remains somewhat ill-defined. West and co-workers defined the term as the combined use of two (or more) retention mechanisms in a single chromatographic system. Adding to this, Gilar and co-workers further define the term as the use of multiple dominant retention mechanisms (5). The dominant designation is an important one as all chromatographic systems involve at least some minor contributions from retention mechanisms other than the primary ones. These minor, but usually important, contributions are often referred to as secondary interactions. For the purposes of this column, we will refer to mixed-mode chromatography where multiple retention mechanisms play a dominant role in the overall chromatographic performance. Mixed-mode systems may be achieved via a number of different routes. However, the combination of partition (RPLC or HILIC) chromatography and ion-exchange seems to be associated most closely with the term.
In their description of new phases that combined partitioning and anion-exchange chromatography, Pohl and Liu noted that mixed-mode columns address a number of key challenges in chromatographic method development (6). Using reversed-phase columns only can result in poor polar analyte retention, basic analyte peak tailing, and column “dewetting” under high aqueous mobile phase content. They go on to note that ion-exchange can be used to tackle some of these issues, but ion-exchange alone is not suitable to separate molecules based on their differences in hydrophobicity. The combination of the two modes of chromatography often provides the potential to address a number of analytical challenges. Mixed-mode chromatography has played a significant role in modern chromatographic practices and has been applied to areas such as oligonucleotides, peptides, proteins, metabolomics, pharmaceutical analysis, and natural product studies, among many others (7).
In an attempt to take an even more recent pulse on the level of interest and utilization of mixed-mode chromatography, a non-exhaustive search of publications in the past year along with a perusal of well-known column vendor websites was conducted. The limited search revealed a significant number of papers and technical notes in this realm, indicating continued interest. What follows is a brief synopsis of what was found, broken down into fundamental studies, new stationary phase designs, and applications of the technology.
Fundamental Research
The application of mixed-mode chromatography is often limited, because of the complexity of the resulting chromatography. With more mechanisms playing a significant role, additional parameters that control these mechanisms need to be taken into account during method development and practice of the resulting procedures. In addition, it is often difficult to find a full description of surface chemistry from vendors or detailed characterization results (8). Fundamental studies that provide the user with information to help guide method development and understand both the potential and limitations of stationary phases is of utmost importance.
Lämmerhofer and associates used chromatographic, molecular modeling and electrochemical techniques to characterize chiral zwitterionic materials (7). Although the target phases were commercialized for use in chiral separations, the group notes significant achiral utility. The columns studied were described as cinchona carbamate stationary phases decorated with cyclohexylsulfonic acid and carbamate residues and are thus likely to provide multiple dominant retention mechanisms. Indeed, the group found the phases to exhibit moderate hydrophobicity, the potential for HILIC operation, and ion-exchange character. A unique aspect of this research was that investigators used a sequential building and analysis of intermediary versions of the stationary phases to better understand the individual structural contributors to retention mechanisms.
Gilar and co-workers investigated the chromatographic attributes of several commercially available columns, including single-mode as well as a recently launched mixed-mode column combining C18 and anion-exchange chemistry (5). The group focused their efforts on understanding the ion-exchange interaction potential of the columns across a wide pH range. Fundamental information regarding the interactions available across a number of different conditions is important to method developers for both selecting and utilizing various stationary phases. For instance, the researchers were surprised to find that the ion-exchange interactions for the mixed-mode phase dropped off at a pH value much lower than the ligand pKa value. The observation was attributed to the impact of ionized surface silanols on the overall surface charge and demonstrates both the complexity and the need to carefully study mixed-mode systems.
New Stationary Phase Research
The majority of the published research over the past year has focused on the development of new stationary phases and their characterization. What follows is not intended to be an exhaustive list, but it should provide an overview of the types and breadth of stationary phases being investigated across the globe in this field.
Shields and Webber reported on the development of a mixed-mode, reversed-phase cation-exchange system based on a thiolyne reaction. The intent was to develop a mixed-mode column with low pH stability. The authors used the separation of monoamine neurotransmitters to demonstrate both the ion-exchange and partition properties of the phase (9). Guo and others published a paper describing the interesting combination of MOF-235, polyethylene glycol, and silica, in a core–shell-based format. The authors indicate a combination of HILIC and ion-exchange is possible with the phase composition (10).
Li and associates described the use of modified dialdehyde cellulose as a substrate for developing stationary phases that exhibit both HILIC and ion-exchange properties. The authors note facile functionalization and suggest this approach as the basis for further stationary phase development (11).
Several stationary phase developments have been recently published by Wang and co-workers. The authors describe a poly(ethyleneimine) embedded N-acetyl-L-phenylalanine stationary phase that is shown to exhibit RPLC, HILIC, and ion- exchange characteristics (12). In a second paper from the group, a stationary phase co-modified with N-isopropylacrylamide and aminophenylboronic acid is described.
The resulting phase was also characterized as providing both hydrophobic and hydrophilic retention capabilities along with anion-exchange properties (13). Wang and associates also reported on the incorporation of ionic liquids with C18 and cyclodextrin moieties bonded to silica supports as potential stationary phases for liquid chromatography (LC) (14). The group characterized the phases as exhibiting hydrophobic and ion-exchange character as well as the potential for use in the HILIC domain. A positive comparison with commercially available, single-mode columns was provided.
Heydar and Hosseini published a paper describing the preparation of a novel, silica-based stationary phase using 9-methylacridine and 9-undecylacridine (15). The phases were shown to exhibit hydrophobic and hydrophilic partitioning as well as anion-exchange characteristics. Finally, Wolrab and associates investigated a series of zwitterionic and strong cation-exchange based mixed-mode phases under RPLC, HILIC, and supercritical fluid chromatography (SFC) conditions (16). The authors noted that ion-exchange interactions appear to prevail over others for the phases studied. However, other interactions, such as partitioning, can be enhanced or attenuated using various mobile phase conditions.
It is clear from the number of reports over a short period of time, as well as from the diversity of approaches, that interest in developing mixed-mode chromatography stationary phases continues.
Applications
Mixed-mode chromatography is powerful, but complex. As noted above, when multiple dominant retention mechanisms are invoked, many variables must be controlled to produce a robust and repeatable method. Mixed-mode chromatography is therefore most often applied only to complex systems. The following are examples of mixed-mode applications found in recent literature.
Artificial Sweeteners in Surface Waters
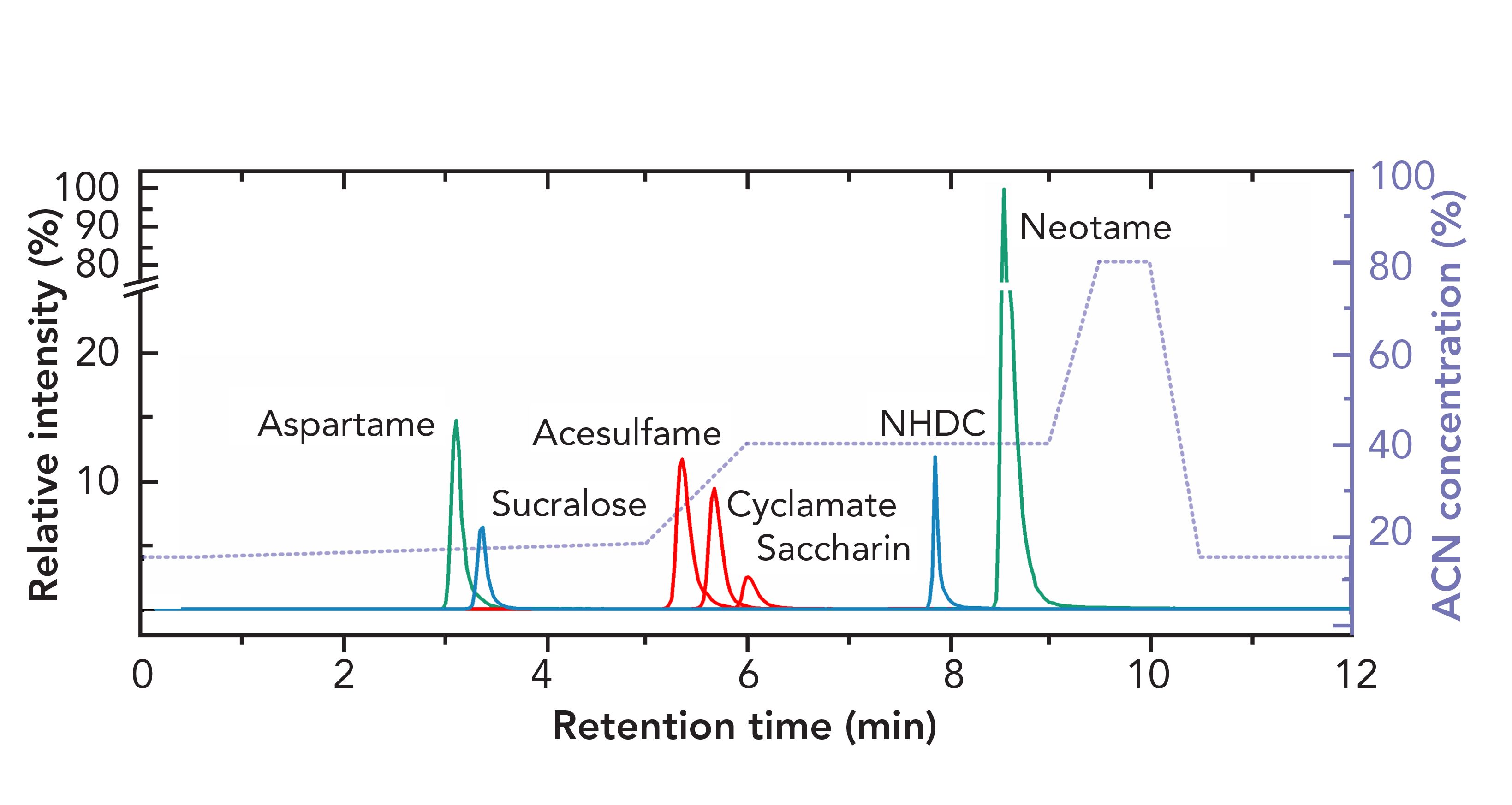
The combination of anion-exchange and RPLC was used to analyze artificial sweeteners in surface waters. Anionic sulfamates (acesulfame, cyclamate, saccharin), zwitterionic dipeptides (aspartame, neotame), and polar derivatives of natural products (sucralose, neohesperidin dihydrochalcone [NHDC]) were all efficiently separated on an octadecylsilane (C18)-strong anion exchanger (SAX) combination phase, as shown in Figure 1. One of the major advantages of the mixed-mode approach noted by the authors was the ability to inject large volumes of sample, presumably because of the accumulation effect of the ion-exchange character (17).
FIGURE 1: Optimized separation of seven artificial sweeteners on a mixed-mode stationary phase. Reproduced with permission from reference (17). ACN is acetonitrile.
Separation of Oligonucleotides
Zhang and associates recently reported on the use of mixed-mode chromatography for the analysis of oligonucleotides. The use of ion-pair reversed-phase chromatography (IP-RPLC) appears to be the most heavily employed mode of separation for oligonucleotides to date. However, anion-exchange, HILIC, and mixed-mode methods have also been successful. According to the authors, the use of mixed-mode separations for oligonucleotides dates back to the early 1980s. The authors also note that RPLC and ion-exchange mixed-mode approaches have displayed improved separations over either mode alone. In addition, it is speculated that multidimensional liquid chromatography (mLC) is poised to deliver much of what mixed-mode chromatography can do and is expected to continue to grow in this space (18). Lämmerhofer and co-workers also proposed the use of a chiral zwitterionic phase, which exhibits both partition and anion-exchange properties, for the first dimension separation of oligonucleotides using RPLC as the second dimension (19).
Underivatized Amino Acids
The application of mixed-mode chromatography for the separation of underivatized amino acids was demonstrated by Moussa and associates (20). The traditional amino acid analysis routine consists of an ion-exchange based separation followed by reaction with ninhydrin or similar reagent. The traditional approach is noted as being time-consuming, nonspecific, and requires a dedicated analyzer. The authors used a “trimodal” column consisting of anion-exchange, cation-exchange, and hydrophobic properties to separate 52 amino acid-related compounds in 18 min with minimal sample preparation. The authors noted that the bimodal systems investigated did not provide the necessary separation of critical pairs.
Deamidation of Proteins
Sze and co-workers reported on the utility of employing ion-exchange in combination with reversed-phase and HILIC separations for the improved analysis of deamidation in proteins (21). The authors note that deamidation of proteins results in several species of very similar hydrophobicities that are thus difficult to separate using partition chromatography alone. The authors suggest that the use of electrostatic interactions—coupled with HILIC chromatography, along with improved sample preparation and advanced mass spectrometric techniques—will help serve to fill deficiencies in this important area of research.
Pentacyclic Triterpenoids
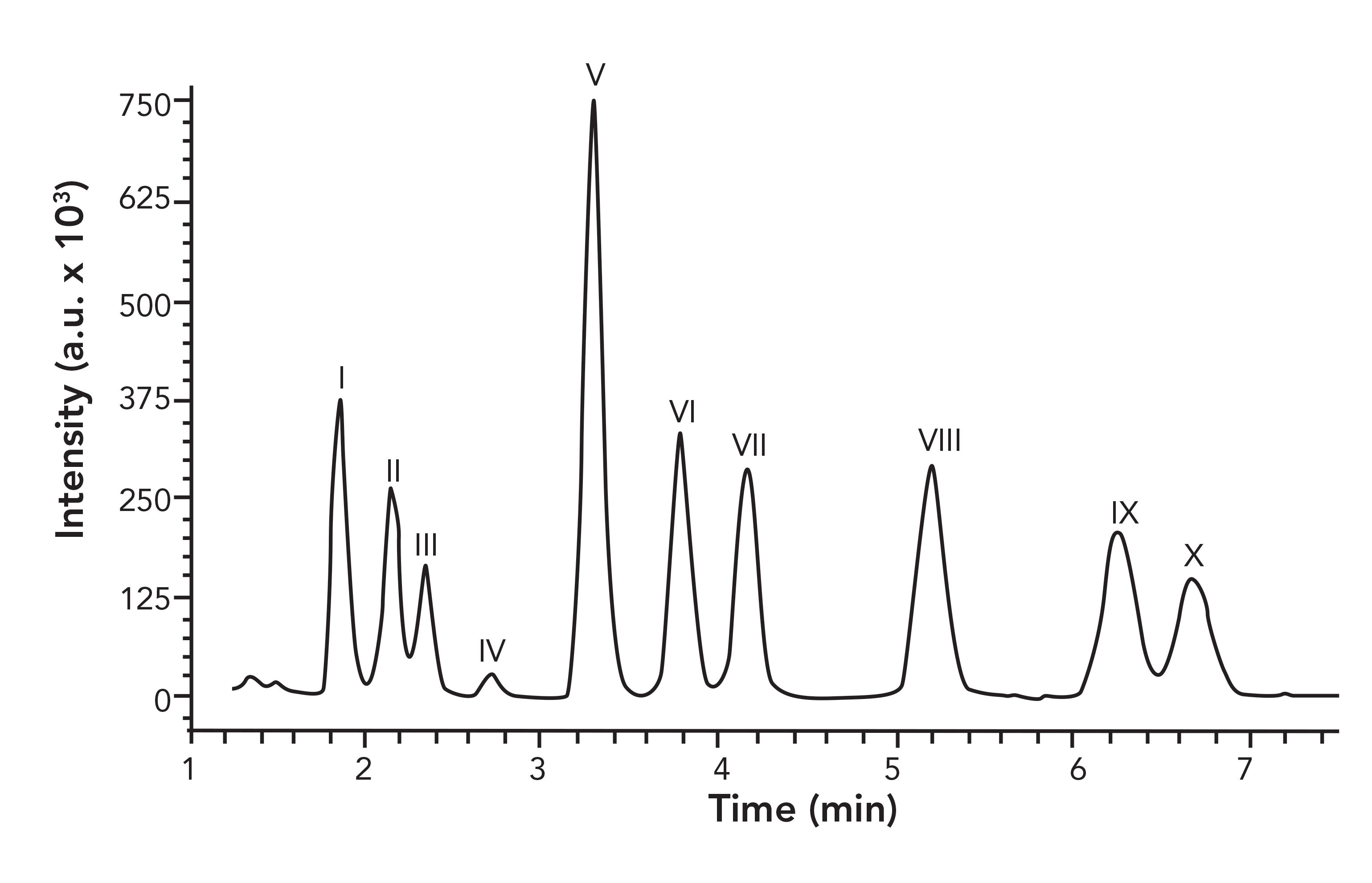
Another application of mixed-mode chromatography published in 2020 tackled the separation of pentacyclic triterpenoids using a mixed-mode, weak-anion-exchange stationary phase (22). The critical pairs of erythrodiol and uvaol, as well as oleanolic acid and ursolic acid, were only resolved with a combination of reversed-phase or HILIC with ion-exchange mechanisms, whereas both RPLC and HILIC alone were found to be insufficient. As shown in Figure 2, the mixed-mode column utilized provided the separation of 10 analytes in approximately a 7-min run time.
FIGURE 2: Optimized separation of pentacyclic triterpenoids on a mixed-mode stationary phase. I is betulin, II is erythrodiol, III is uvaol, IV is friedelin, V is lupeol, VI is β-amyrin, VII is α-amyrin, VIII is betulinic acid, IX is oleanolic acid and X is ursolic acid. Note a.u. is arbitrary units. Reproduced with permission from reference (22).
It should also be noted that many vendors provide application data in support of the use of mixed-mode phases. A cursory search through websites of many prominent vendors showed recent activity in this realm. The reader is encouraged to visit column vendor sites for more information.
Conclusions
A non-exhaustive literature search and perusal of web-based information revealed that interest in mixed-mode chromatography is alive and well. Recent applications in environmental, pharmaceutical, and biopharmaceutical areas demonstrate that the use of multiple dominant retention mechanisms continues to assist researchers in meeting the need for complex separations. The breadth of research toward the development of new mixed-mode stationary phases and their subsequent characterization over the past year indicates there is significant space for, and interest in, new discoveries. Finally, fundamental research and characterization of commercially available columns is paramount and is expected to greatly facilitate the intelligent use of these powerful stationary phases.
References
(1) D.S. Bell, LCGC North Am. 38(4), 211–219 (2020).
(2) D.S. Bell, LCGC North Am. 37(4), 232–243 (2019).
(3) C. West, et al., LCGC North Am. 30(6s), 22–33 (2017).
(4) K. Zhang and X. Liu, J. Pharm. Biomed. 128, 73–88 (2016).
(5) Z. Kadlecová, et al., J. Chromatogr. A 1625, 461301 (2020).
(6) X. Liu and C. Pohl, A Weak Anion-Exchange/Reversed-Phase Mixed-Mode HPLC column and its Applications. Am. Lab. 2007; Available from: https://www.americanlaboratory.com/913-Technical-Articles/1454-A-Weak-Anion-Exchange-Reversed-Phase-Mixed-Mode-HPLC-Column-and-its-Applications/.
(7) M. Ferri, et al., J. Chromatogr. A 1621, 461075 (2020).
(8) R. Karongo, et al., J. Chromatogr. A 1627, 461430 (2020).
(9) E.P. Shields and S.G. Weber, J. Chromatogr. A 1618, 460851 (2020).
(10) T. Si, et al., Anal. Chim. Acta. 1116, 62–69 (2020).
(11) J. Gao, et al., J. Chromatogr. A 1618, 460885 (2020).
(12) M. Wan, et al., Microchem J. 157, 105021 (2020).
(13) D. Zhou, et al., J. Chromatogr. A 1627, 461423 (2020).
(14) J. Zhou, et al., J. Chromatogr. A 1634, 461674 (2020).
(15) E.S. Hosseini and K.T. Heydar, Talanta 221, 121445 (2021).
(16) D. Wolrab, et al., J. Chromatogr. A 1635, 461751 (2021).
(17) J. Henschel and H. Hayen, MethodsX 7, 101134 (2020).
(18) A. Goyon, P. Yehl, and K. Zhang, J. Pharm. Biomed. 182, 113105 (2020).
(19) F. Li, et al., J. Chromatogr. A 1625, 461338 (2020).
(20) A. Desmons, et al., J. Chromatogr. A 1622, 461135 (2020).
(21) S.K. Sze, G. JebaMercy, and S.C. Ngan, Methods 3, 215–233 (2020).
(22) D.I. Falev, et al., J. Chromatogr. A 1609, 460458 (2020).
About The Author

David S. Bell is a director of Research and Development at Restek. He also serves on the Editorial Advisory Board for LCGC and is the Editor for “Column Watch.” Over the past 20 years, he has worked directly in the chromatography industry, focusing his efforts on the design, development, and application of chromatographic stationary phases to advance gas chromatography, liquid chromatography, and related hyphenated techniques. His main objectives have been to create and promote novel separation technologies and to conduct research on molecular interactions that contribute to retention and selectivity in an array of chromatographic processes. His research results have been presented in symposia worldwide, and have resulted in numerous peer-reviewed journal and trade magazine articles. Direct correspondence to: LCGCedit@mmhgroup.com

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
New Study Investigates Optimizing Extra-Column Band Broadening in Micro-flow Capillary LC
March 12th 2025Shimadzu Corporation and Vrije Universiteit Brussel researchers recently investigated how extra-column band broadening (ECBB) can be optimized in micro-flow capillary liquid chromatography.









