Online Hydrophobic Interaction Chromatography–Mass Spectrometry (HIC–MS) Analysis of Proteins
Recent advances in stationary phases for hydrophobic interaction chromatography (HIC) permit HIC–MS analysis of intact antibodies and other proteins using direct flow to the mass spectrometer.
Hydrophobic interaction chromatography (HIC) is widely used for protein analysis. Its use of nonvolatile salts has precluded direct flow to mass spectrometers. Recent advances in stationary phases have overcome that obstacle and permit HIC–MS analysis of intact antibodies and other proteins.
Hydrophobic interaction chromatography (HIC) is the chief alternative to ion-exchange chromatography (IEC) for the nondenaturing analysis of proteins. Separation by ion-exchange is based on difference in electrostatic charge, while separation by HIC is based on polarity differences, generally involving the hydrophobic residues. Its selectivity is then complementary to that of ion-exchange. Polarity differences are also the basis of separation in reversed-phase liquid chromatography (RPLC), but because the conditions of RPLC denature proteins, then the two modes, RPLC and HIC, have access to different sets of hydrophobic residues. As a result, their selectivity differs as well. HIC has emerged as the chief mode used for analysis of antibody–drug conjugates (ADCs) in which the ligands are linked through cysteine residues (1,2); an example is presented in Figure 1 (3).
FIGURE 1: Antibody–drug conjugate variants separated via HIC. The numbers denote the number of cysteine residues derivatized with the conjugate. Figure adapted from reference (3).
Recent advances in technology have made it feasible to analyze large intact proteins by mass spectrometry. These advances have created a demand in top-down proteomics for separation methods that do not denature proteins. The reason is that the mass spectra of proteins with native structure have lower charge states and are appreciably less complex than those of denatured proteins, and therefore, they are easier to deconvolute. Conventional HIC is eliminated from consideration. While it preserves structure, it also involves gradients beginning with high concentrations of nonvolatile salts such as ammonium sulfate. Ammonium acetate is a volatile salt, but retention in HIC comparable to the level obtained with ammonium sulfate would require 3–4 M of ammonium acetate, a level that would clog the orifice of current mass spectrometers (4). A recent study (5) took note of the fact that the more hydrophobic a HIC material was, the less salt was required for retention. Accordingly, the ligand in the HIC coating was expanded beyond the usual propyl- or butyl- to encompass everything from pentyl- to decyl- ligands. The pentyl- and hexyl- ligands proved to confer adequate retention for small proteins starting with 1 M ammonium acetate, a concentration that a mass spectrometer can handle (Figure 2). This approach also works well for proteins as large as monoclonal anti- bodies (mAbs) (Figure 3) (6).
FIGURE 2: HIC–MS of lysozyme on a PolyHEXYL A capillary. (a) The total ion chromatogram (TIC). (b) Zoom-in on mass spectrum of charge state 8+, showing the correct mass for lysozyme. (c) Mass spectrum. Figure adapted from reference (5).
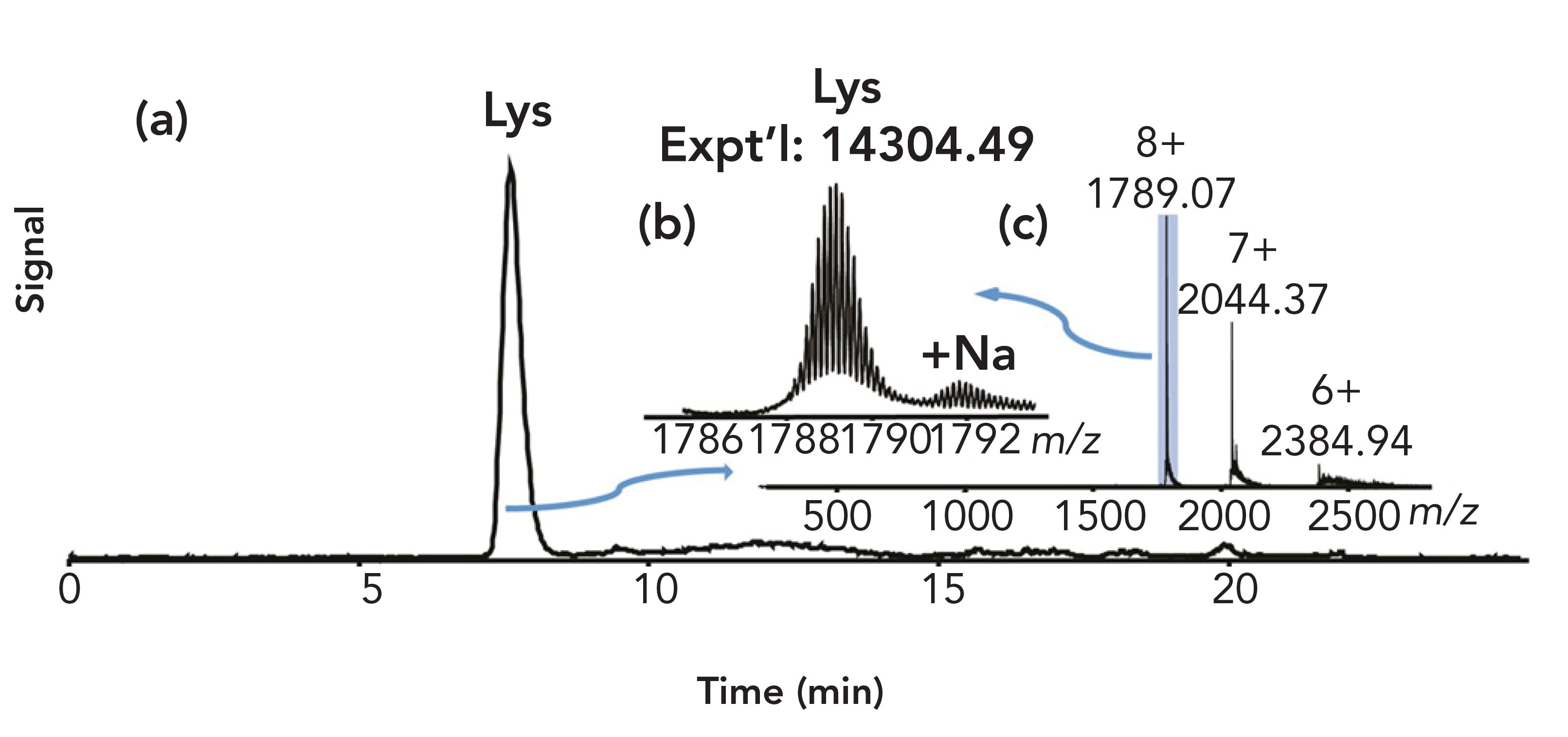
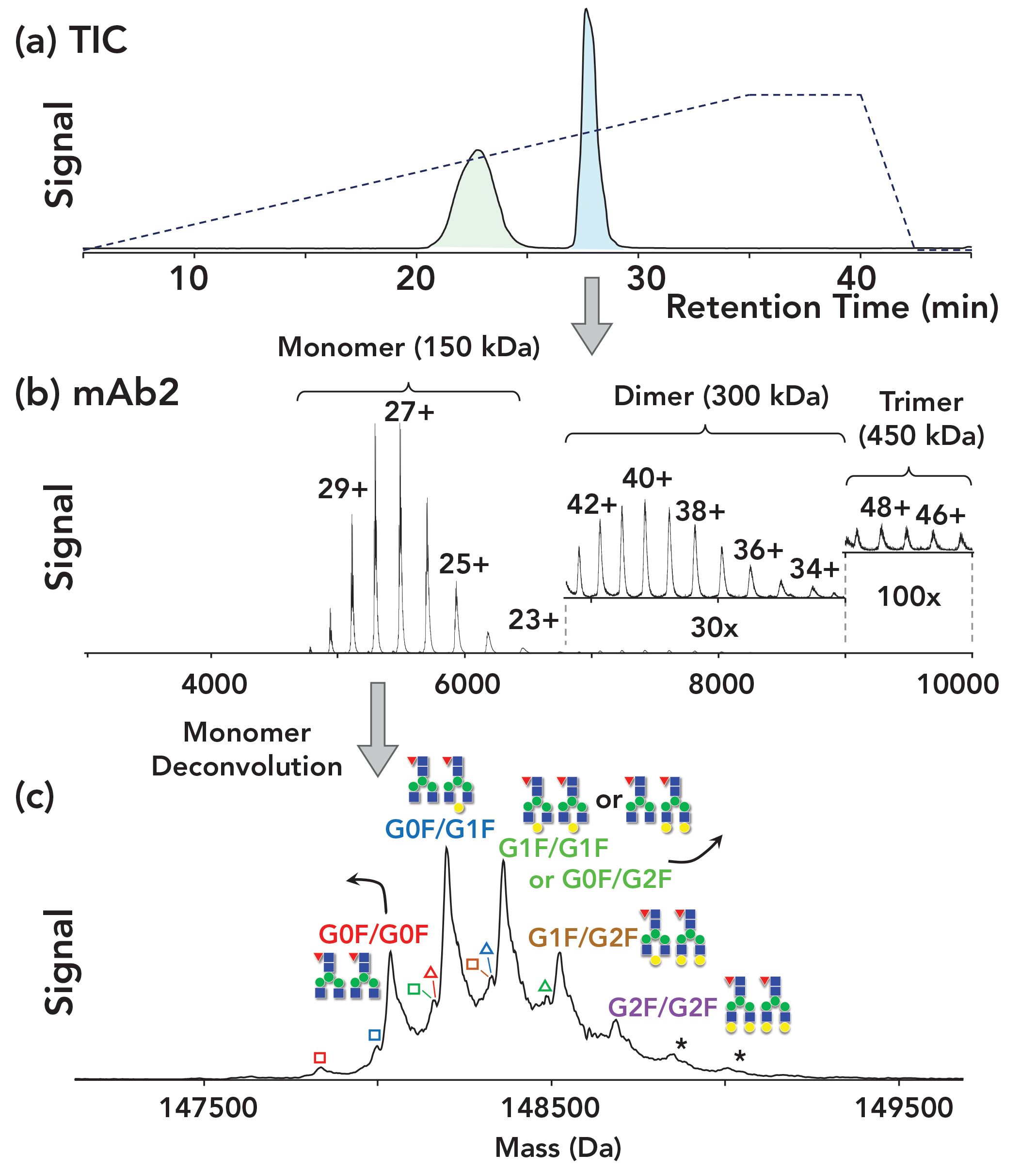
FIGURE 3: Analysis of two mAbs on a PolyPENTYL A capillary. The mAb eluting at 28 min (mAb2) is the NIST standard antibody. Its dimer and trimer are readily evident in the mass spectrum, and with deconvolution, the glycosylation variants are evident as well. The distribution of these variants matches that reported in the scientific literature. a) Total ion chromatogram. b) The mass spectrum of mAb2; the inserts show zoom-ins for the dimer and trimer. c) The deconvoluted mass spectrum of mAb2 monomer, annotated to identify the glycosylation variants. Figure adapted from reference (6).
The selectivity and resolution obtained with the new columns and mobile phases compare favorably with those obtained with columns and running conditions used for conventional HIC, as seen in Figure 4.
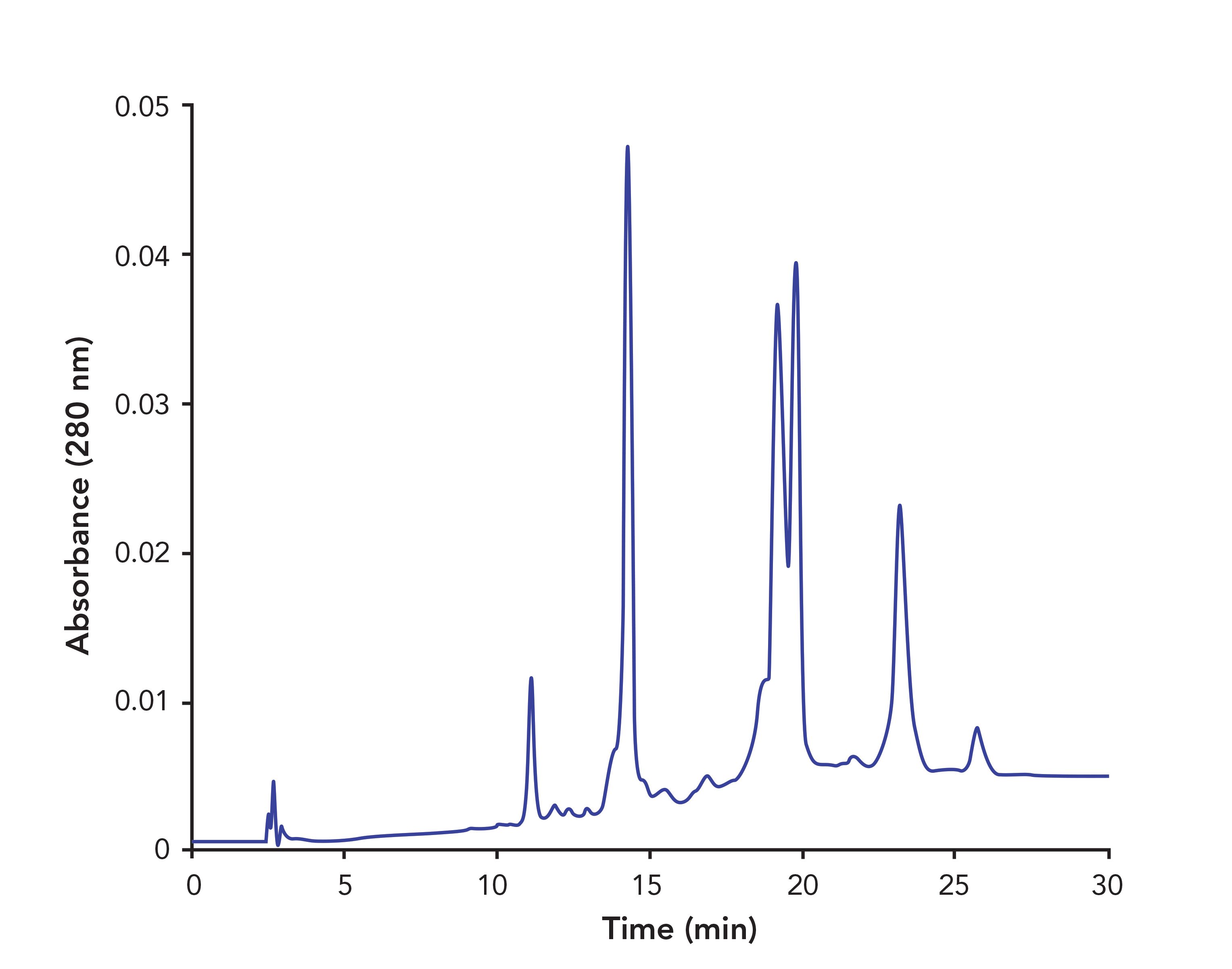
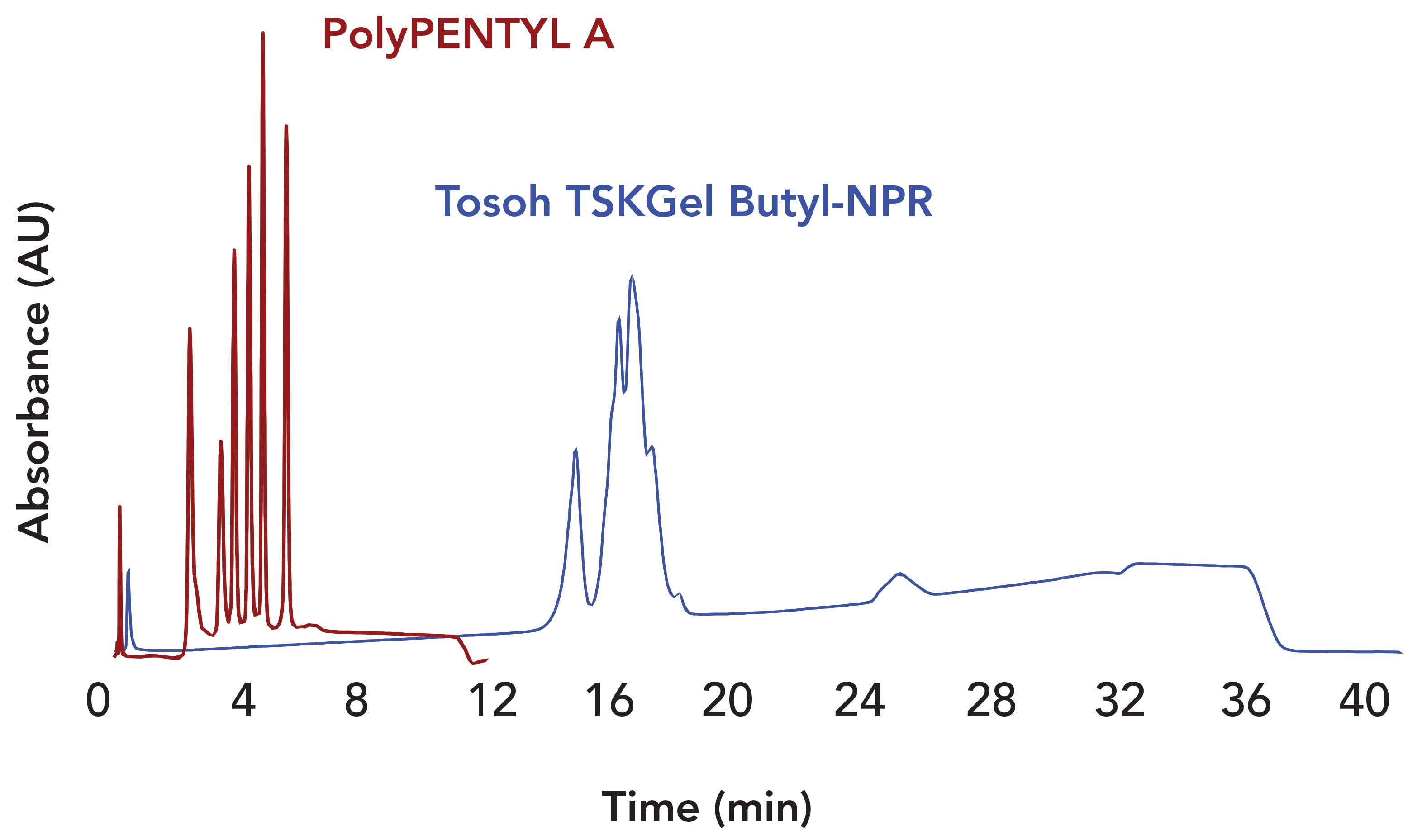
FIGURE 4: Comparison of the separation of a mixture of six antibodies. Temperature: Ambient. PolyPENTYL A column: 50 x 2.1-mm, 3-μm, 1000-Å pore diameter. Mobile phase A:1 M ammonium acetate, pH 7.0. Mobile phase B: 20 mM ammonium acetate with 50% acetonitrile, pH 7.0. Gradient: 10 min, 0–100% B. Flow rate: 1 mL/min. TSKgel Butyl-NPR column: 35 x 4.6-mm, 2.5 μm. MP A: 1.5 M ammonium sulfate + 500 mM arginine + 20 mM Na-phosphate, pH 7.5. MP B: Same without ammonium sulfate, pH 7.5. Gradient: 45 min, 0–100% B. Flow rate: 0.5 mL/ min. The PolyPENTYL A data was adapted from reference (7). The TSKgel Butyl-NPR data was a personal communication of Madhavi Srikoti, Bristol Myers Squibb, New Brunswick, NJ.
For most proteins, an appropriate gradient runs from a high level of ammonium acetate, typically in the range 0.7–1.0 M, to a low concentration plus 50% organic solvent (either acetonitrile or propanol). One recurring question about this method is why the organic solvent doesn’t denature the proteins. The answer presumably lies in the kinetics involved. When left in the final mobile phase for hours, most susceptible proteins probably would denature. For that matter, leaving a protein sitting in a conventional HIC column for hours also leads to its denaturation (8). Under the conditions used here, the kinetics of chromatography are faster than the kinetics of denaturation, and proteins enter the orifice of the mass spectrometer with their structure intact. One reason for the durability of the protein structure may lie in the salt used. High concentrations of conventional HIC salts, such as ammonium sulfate, strip away a protein’s sphere of hydration. No longer solvated by the medium, the protein tends to partition into another phase, either via self-association as a solid (“salting out”) or via adsorption onto a HIC stationary phase surface. Acetate salts tend to have a protective effect on the sphere of hydration (9), and may play a role in the ruggedness of the protein’s retention of native structure. Such speculation can be assessed through the effects of using lower acetate concentrations. Figure 5 examines the consequences with lysozyme.
FIGURE 5: Lysozyme on a PolyPENTYL A column. Mobile phases: (a) 2.5 M ammonium acetate; (b) 20 mM ammonium acetate; (c) 20 mM ammonium acetate with 50% acetonitrile. Adapted from reference (5).
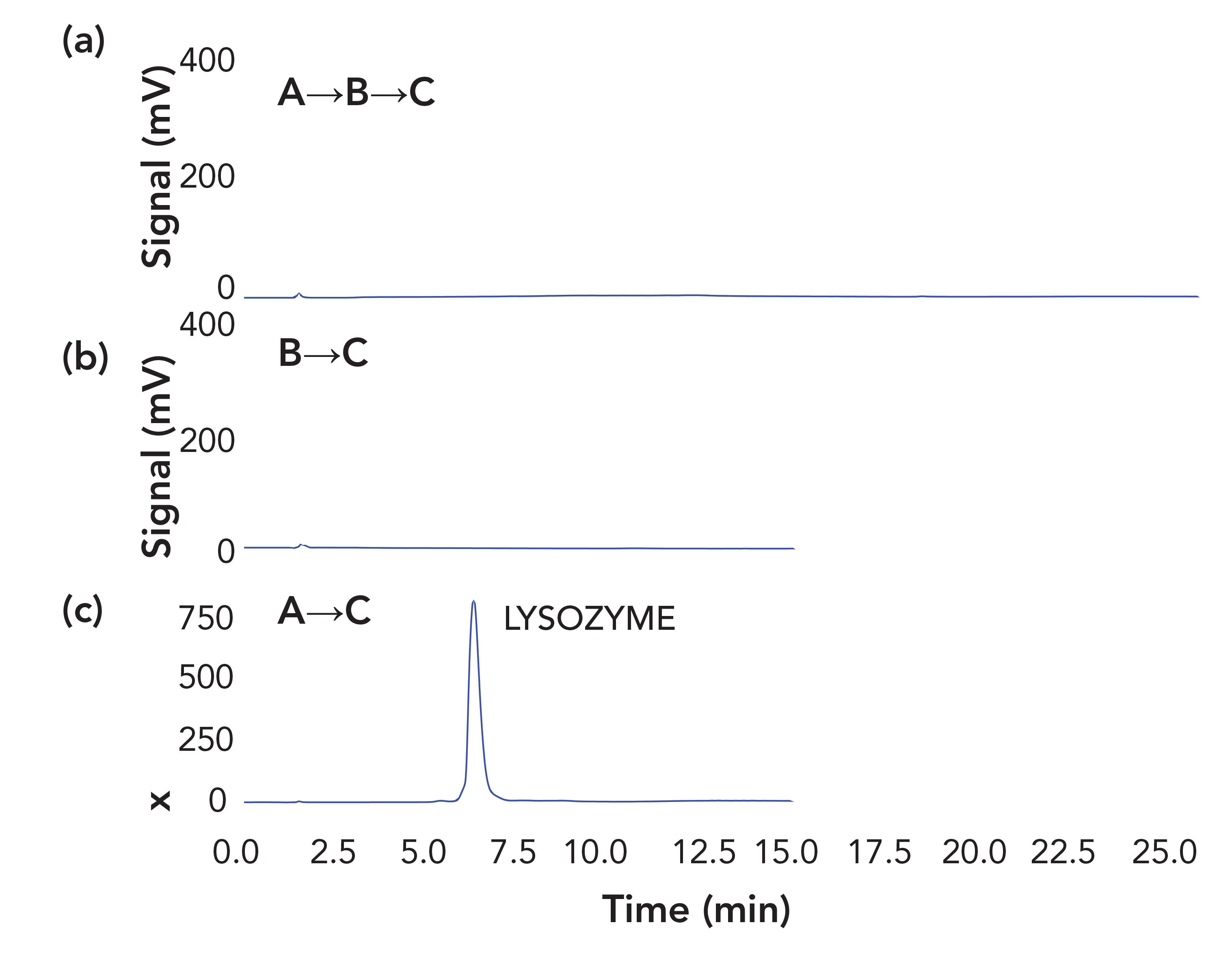
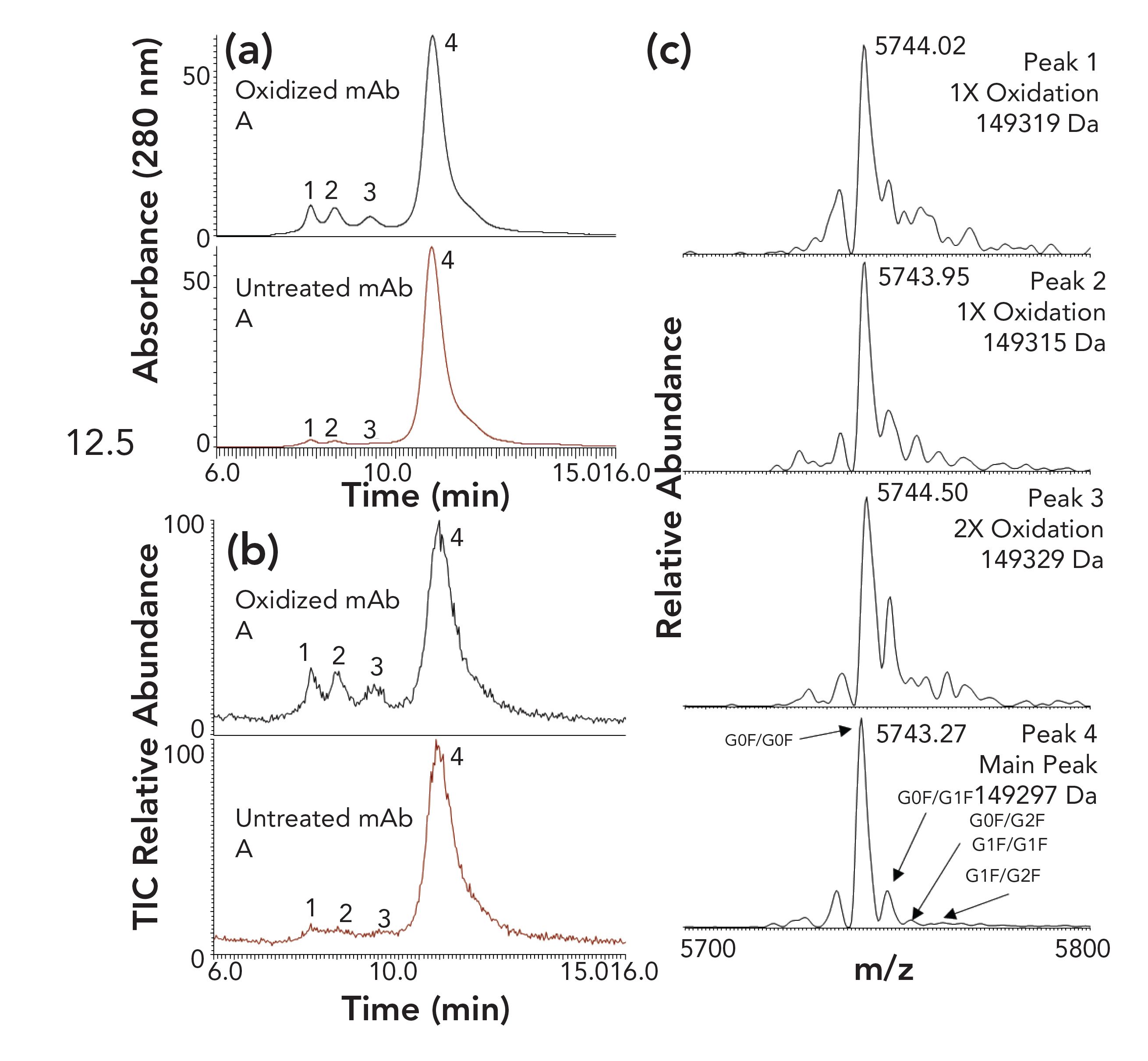
The top and middle panels involved conditions where lysozyme was in 20 mM ammonium acetate prior to introducing acetonitrile. The low concentration of acetate presumably left hydrophobic patches on the protein’s surface less than fully hydrated, making them more accessible to the stationary phase. A subsequent gradient to 50% acetonitrile failed to elute the protein in a reasonable time frame. By contrast, when the protein was exposed to acetonitrile while there was still a high concentration of acetate present, access to the hydrophobic patches was limited and the protein was eluted readily with good peak shape. This subject has been further explored in two subsequent papers. In the first, a group at Genentech took advantage of the greater retention at low concentrations of acetate to optimize conditions for analysis of variants of mAbs (10). A combination of a less-hydrophobic stationary phase (PolyPROPYL A) and 150 mM ammonium acetate permitted the isocratic separation of a mAb from a number of its variants, as seen in Figure 6.
FIGURE 6: Analysis of mAb variants on a PolyPROPYL A column. Mobile phase (isocratic): 150 mM ammonium acetate. a) Oxidized mAb (top) and untreated mAb (bottom); UV detection. b) Overlaid total ion chromatograms of the same two figures as in a). c) Expanded mass spectra of the most abundant charge state (26+) and deconvoluted masses of peaks 1 and 2 (1x oxidation), peak 3 (2x oxidation), and peak 4 (main peak). Adapted from reference (10).
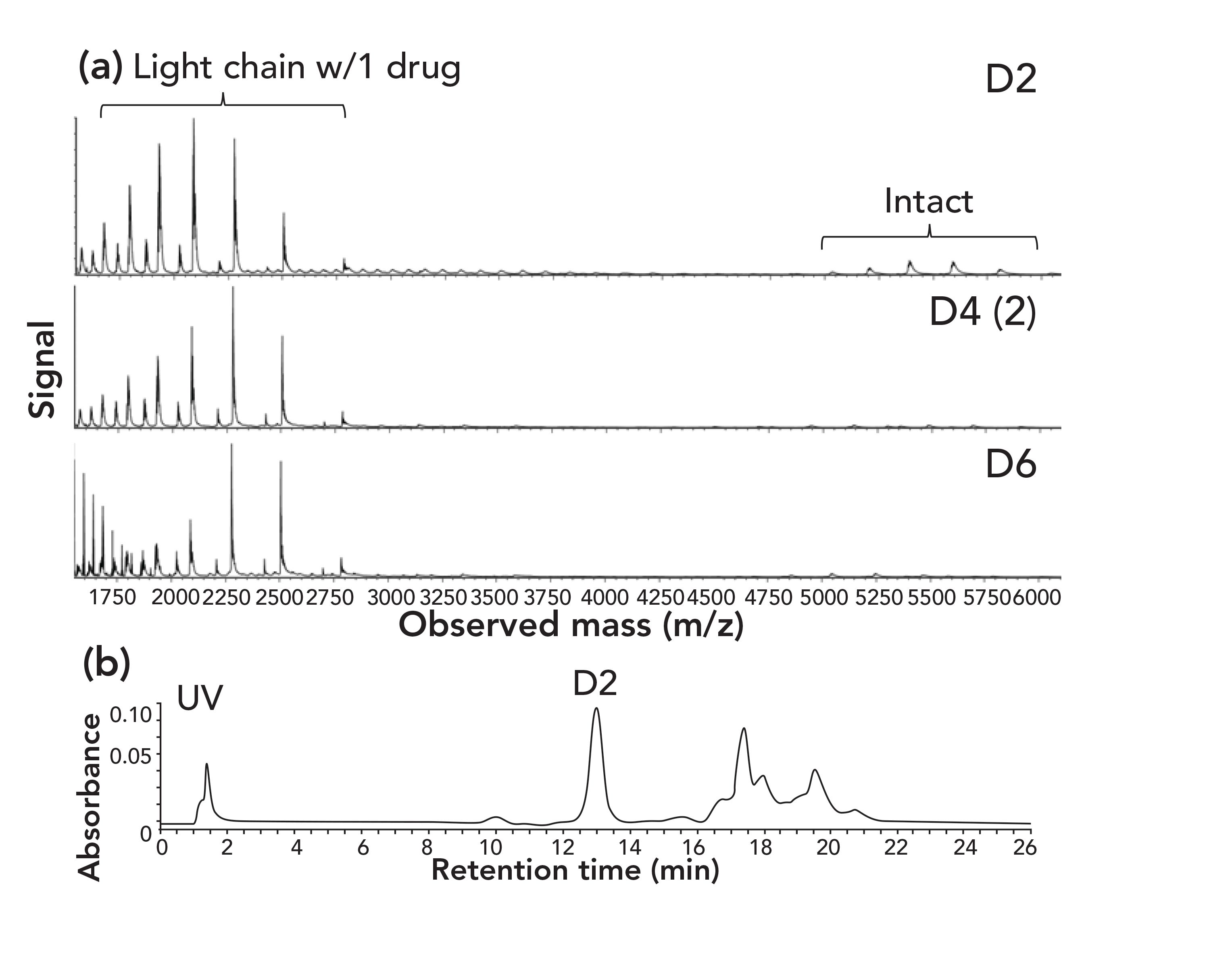
In the second study, Chen and others (11), offer additional insight into the impact of these running conditions on the stability of protein structure. Silica particles were given a coating of methyl methacrylate and then used for analysis of two ADCs under conditions similar to those described above for HIC–MS. The authors used a gradient to 50% 2-propanol with a constant concentration of 50 mM ammonium acetate; these conditions were termed native reversed-phase liquid chromatography (nRPLC). This term is as acceptable as HIC–MS; the HIC conditions described above do seem to obliterate the boundaries between HIC and RPLC. The ADCs were eluted in a profile typical of cysteine-linked ADCs in conventional HIC, judging from the ultraviolet (UV) absorbance traces (Figure 6b). However, the mass spectra of the variants of the ADC (Figure 7a) exhibit considerable dissociation of noncovalently attached light chains from the antibody complex. Dissociation to this extent is not evident in the UV trace. Evidently, these conditions neither denatured the original antibody (the DAR-0 form) nor was the original antibody dissociated from the non-covalent ADC complex evident in the UV trace. However, in the presence of a low concentration of ammonium acetate and a high concentration of organic solvent, the complex was sufficiently fragile that dissociation occurred from the greater stress encountered at the orifice of the mass spectrometer, which indicates that the ruggedness of structure of proteins, such as a mAb or a noncovalent complex, involves a balance of factors. Successful MS analysis of noncovalent complexes will presumably require further optimization of the chromatography conditions.
FIGURE 7: Analysis of brentuximab vedotin (Adcetris) on a column of silica with a coating of polymethyl methacrylate. (a) Mass spectra of ADC DAR-2, DAR-4 and DAR-6 variants obtained with a Xevo G2 qTOF mass spectrometer. (b) UV absorbance trace. Adapted from reference (11).
Summary
These new combinations of stationary and mobile phases combine the protein compatibility and high resolution of HIC with the ever-increasing capabilities of mass spectrometry. HIC–MS promises the analysis of intact proteins online, without the delay, inconvenience, and potentially incomplete sequence coverage of middle-down or bottom-up approaches to proteomics.
Acknowledgments
Adcetris is a trademark of Seattle Genetics. TSKgel Butyl-NPR is a trademark of Tosoh Corporation. PolyPROPYL A, PolyPENTYL A, and PolyHEPTYL A are trademarks of PolyLC Inc.
References
(1) B. Bobály, S. Fleury-Souverain, A. Beck, J.-L. Veuthey, D. Guillarme, and S. Fekete, J. Pharm. Biomed. Anal. 147, 493–505 (2018).
(2) L.N. Tumey, Ed., Antibody-Drug Conjugates (Meth. Molec. Biol. 2078), (Humana Press, New York, New York, 2020).
(3) J. Guo, S. Kumar, M. Chipley, O. Marcq, D. Gupta, Z. Jin, D.S. Tomar, C. Swabowski, J. Smith, J.A. Starkey, and S.K. Singh, Bioconj. Chem. 27, 604–615 (2016).
(4) Y. Yan, T. Xing, S. Wang, T.J. Daly, and N. Li, J. Pharm. Biomed. Anal. 186, 113313 (2020)
(5) B. Chen, Y. Peng, S.G. Valeja, L. Xiu, A.J. Alpert, and Y. Ge, Anal. Chem. 88, 1885–1891 (2016).
(6) B. Chen, Z. Lin, A.J. Alpert, C. Fu, Q. Zhang, W.A. Pritts, and Y. Ge, Anal. Chem. 90, 7135–7138 (2018).
(7) M. Srikoti, M.S. Bolgar, and Y. Kazakevich, J. Chromatogr. B, 1140, 121984 (2020).
(8) E. Haimer, A. Tscheliessnig, R. Hahn, and A. Jungbauer, J. Chromatogr. A, 1139, 84–94 (2007).
(9) J.L. McNay, J.P. O’Connell, and E.J. Fernandez, Biotechnol. Bioeng. 76, 233–240 (2001).
(10) B. Wei, G. Han, J. Tang, W. Sandoval, and Y.T. Zhang, Anal. Chem. 91, 15360–15364 (2019).
(11) T.-H. Chen, Y. Yang, Z. Zhang, C. Fu, Q. Zhang, J.D. Williams, and M.J. Wirth, Anal. Chem. 91, 2805–2812 (2019).
Andrew J. Alpert is the president of PolyLC Inc., in Columbia, Maryland. Direct correspondence to: aalpert@polylc.com.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









