Superficially Porous Particles: Perspectives, Practices, and Trends
LCGC Europe
This review provides an updated overview of the theory behind the success of SPP technology, trends, benefits, and limitations. It also summarizes the latest developments of sub-2-?m SPPs and instrumental constraints associated with their use.
Columns packed with superficially porous particles (SPPs) have created considerable excitement over the last few years. Indeed, this column technology manifests the advantages of fully porous material (loading capacity, retention) and some beneficial properties of nonporous particles (kinetic performance). This review provides an updated overview of the theory behind the success of SPP technology, trends, benefits, and limitations. It also summarizes the latest developments of sub-2-µm SPPs and instrumental constraints associated with their use. Finally, it describes several applications to illustrate the performance and the universal applicability of these newly engineered particles.
Superficially porous particles (SPPs) (also called core–shell, fused-core shell, partially porous, pellicular, or solid-core) are made of a solid, nonporous silica core surrounded by a porous shell layer with similar properties to those of the fully porous materials used in conventional high performance liquid chromatography (HPLC) columns. The "fused-core" terminology was originally introduced by Jack Kirkland to describe the manufacturing procedure that "fuses" a porous silica layer onto a solid silica particle (1).
The very high efficiency achieved on columns packed with sub-3-µm SPPs, combined with convenient operating conditions (modest back pressures and the ability to use conventional HPLC instruments), has generated significant interest in the chromatographic community and widespread applications in many fields (2). Columns packed with sub-3-µm SPPs rival the efficiency of columns packed with sub-2-µm fully porous particles, but the former generate only half the back pressure. As a result, practitioners can use such columns on regular HPLC equipment, leading to the initial interest in these materials and their successful application. Moreover, further performance improvement is possible with very fine SPPs (1.3–1.7 µm), though the use of ultralow-dispersion ultrahigh-pressure liquid chromatography (UHPLC) systems (for example, extracolumn peak variance σec2 < 3 µL2) is mandatory. Faster analysis and higher efficiency is always desirable in liquid chromatography (LC), particularly to pharmaceutical scientists or researchers in life science wishing to attain higher productivity in the laboratory or more accurate analysis (higher resolution) of very complex samples. Reducing analysis time while maintaining resolution requires high kinetic performance (more separation power per unit time) using smaller particles, better particle morphology, or both.
The initial intent of applying SPPs was to enhance kinetic performance in the analysis of large biomolecules such as therapeutic proteins. The rationale behind this concept was to improve column efficiency by shortening the diffusion path that molecules have to travel and, thus, improve their mass transfer kinetics (3,4). Shell-type (pellicular) particles were first developed by Horváth and colleagues in the late 1960s for the analysis of large molecules in ion-exchange mode (5). Shortly afterward, Kirkland demonstrated that superficially porous particles (pellicular materials) with 30–40 µm diameters could provide much better separations than totally porous ones (6). In 2001, a new column for fast protein or peptide analysis was introduced that was packed with a 5-µm SPP with a shell thickness of 0.25 µm.
In 2007, a revolution started with the commercialization of a new generation of sub-3-µm SPPs adapted for separation of small and large molecules (7) by Advanced Materials Technology. This material possesses a 1.7-µm solid core covered by a 0.5-µm-thick shell of porous silica. It combined the advantages of both fully porous and nonporous particles. In particular, this improved particle design solved the problem of the low loading capacity of early pellicular particles because an approximately 75% volume fraction of these particles is still porous. Since then, many providers commercialized SPPs with particle sizes ranging between 1.3- and 5-µm. Table 1 provides a current list of commercially available columns packed with SPP materials, their pertinent properties, and available bonded phases.
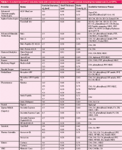
Table 1: A current list of HPLC columns made with commercially available SPP materials including the newest sub-2-µm SPPs.
Column Performance: The Impact of Shell Thickness
In 1956, the famous van Deemter equation was proposed to describe column performance (plate height) as a function of linear velocity (8). Since then, several plate height and rate models have been derived for LC by numerous researchers. Knox suggested a three-term equation to describe the dependency of the theoretical plate height (H) of a column as a function of linear velocity (u) (9):
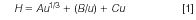
where A, B, and C are constants, determined by the magnitude of band broadening because of eddy dispersion, longitudinal diffusion, and mass transfer resistance, respectively. In practice, the C term is the sum of contributions from resistance to both trans-particle mass transfer (CP) and external film mass transfer (CF).
The application of reduced parameters is common in engineering to compare the performance of columns in normalized units. It is useful to convert H to a dimensionless parameter such as reduced plate height (h = H/dp), where dp is particle diameter. The advantage of this approach is the ability to compare the performance of columns packed with particles of different sizes or structures (morphology). With the use of the reduced parameters, equations similar to equation 1 can be written. Therefore, the total reduced plate height (h) can be written as the sum of at least four different contributions:
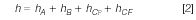
where hA, hB, hCp, and hCF are the reduced plate height contributions from eddy dispersion, longitudinal diffusion, trans-particle mass transfer, and external film mass transfer, respectively.
The initial idea of preparing SPPs was to increase column efficiency by reducing mass transfer resistance across particles (Cp). However, it turned out that trans-particle mass transfer resistance is far from being the main contributor to band broadening in LC for small molecules (especially for modern columns packed with small particles) (3). The enhanced performance of SPP materials clearly lies in contributions from other factors.
Theoretically, the intraparticle diffusivity (the diffusion speed inside the particle pores) of an SPP depends on the ratio of the diameter of the solid core to that of the SPP (dp). As this ratio (ρ) increases, the mass transfer kinetics becomes faster through the particles. Similarly, the external mass transfer (CF) process depends on the particle's structure. It has been demonstrated that mass transfer kinetics is mainly affected by the external film mass transfer resistance across the thin layer of the mobile phase surrounding the external surface area of the particles (3). The trans-particle mass transfer resistance is important for large molecules when the diffusion time inside the particles is much longer, but not so critical for small molecules. As far as diffusion time is concerned, the 2.7-µm Halo (Advanced Materials Technology) and Poroshell 120 (Agilent Technologies) phases are equivalent to a 1.8-µm fully porous packing material, while the 2.6-µm Kinetex material (Phenomenex) is equivalent to a 1.3-µm fully porous stationary phase (10).
Additionally, the presence of a solid core within an SPP has a direct impact on longitudinal diffusion (the B term of the van Deemter equation). It decreases the B term contribution to the plate height by about 20% when the ratio of the core to the particle diameter (ρ) is 0.63 (for example, Halo [Advanced Materials Technology], Ascentis Express [Sigma-Aldrich], BrownLee SPP [PerkinElmer]) (3,4). However, the improvement in overall efficiency is not only because of the reduced internal particle porosity of SPPs: It was experimentally determined that the solid core reduced longitudinal diffusion by about 30% in comparison with fully porous particles (11). This reduction in longitudinal diffusion produces only a ~10% increase in total overall column efficiency compared with columns packed with fully porous particles.
Figure 1(a) shows the theoretical reduction in reduced plate heights (h) on a relative scale for trans-particle mass transfer (hCp), external film mass transfer (hCF), and longitudinal diffusion (hB) as a function of particle structure (ρ) (4). Theoretically, on average about ~40–60% lower h values are expected of current SPPs compared to fully porous materials of the same diameters. Please note that Figure 1(a) does not say anything about the relative contribution of the three different dispersive phenomena to the total h.

Figure 1: The theoretically expected reduction of (a) reduced plate height contributions and (b) the volume fraction of the porous shell as a function of particle structure (Ï). The light blue zones indicate the Ï range of commercially available SPP materials.
Finally, according to several experimental results, eddy dispersion (the A term in the van Deemter equation) in SPP columns is significantly smaller (~30–40%) than that of the columns packed with fully porous particles of similar size (12–14). This is unexpected because particle structure should not affect the multipath dispersion of solutes when travelling through the column. When modern SPP columns are operated at their optimum linear velocity, 50% of the band dispersion results from long-range packing heterogeneity (12–14). However, it is still unclear whether this significant improvement in efficiency is because of narrower particle size distribution (PSD) of SPPs or their higher density, which enables them to form very homogeneous, efficient packed beds. Some recent studies have indeed indicated that SPPs displaying a very narrow PSD can lead to unprecedented low minimal plate heights (3,4). Another explanation proposed was that some SPPs appear to have rougher surfaces than fully porous particles. This might also have a positive influence on packing quality, apart from the narrowness of the PSD.
Based on Figure 1(a), a thinner shell is advantageous for efficiency, but it also reduces sample loading capacity and analyte retention. Therefore, the optimum shell thickness is a compromise between efficiency, sample loading capacity, and analyte retention. The sample loading capacity and expected retention factor of a given solute are proportional to the stationary phase volume. Therefore, sample loading and retention are expected to be somewhat lower on SPPs than on fully porous particles. Figure 1(b) shows the volume fraction of the porous layer of some typical SPP structures. Please note that nominal particle size and shell thickness values were considered for Figure 1; however, in practice, the average and nominal values could be slightly different.
As can be seen in Figure 1(b), the commercially available SPP materials present a huge variety in terms of volume fraction of porous material, between 27% and 75%. SPPs with low volume fractions correspond to relatively large wide-pore particles (for example, 3.6-µm Aeris WP [Phenomenex] or 5-µm Poroshell 300 [Agilent Technologies]) developed for large molecules. In practice, the loadability of most commercially available sub-3-µm SPP materials seems to be similar to that of fully porous particles of the same diameter (15,16). This result may be explained partly by the relatively high surface area of the silica in the porous shell. It is also conceivable that sample molecules do not penetrate appreciably into the centre of totally porous particles (15). Similar to loadability, it appears that the retention characteristic of sub-3-µm SPP materials may not be significantly different from that of their totally porous counterparts when operating under identical conditions (16).
Latest Developments in SPP Technology: Decreasing the Particle Size
The last decade has seen significant enhancements in LC performance brought about by the development of new columns packed with sub-2-µm fully porous particles and improved UHPLC instrumentation. This new level of performance originates from the achievement of lower height equivalent of a theoretical plate (HETP) values, which is manifested in practice as narrower peaks eluted in a very short time and with superior resolution.
As described above, the exceptional performance of columns packed with SPPs with low-molecular-weight analytes is related to a reduction of eddy dispersion and longitudinal diffusion. Because of these unexpected but beneficial features, SPPs are likely to have better morphology for further performance optimization via particle miniaturization (17). Therefore, column providers have introduced very fine sub-2-µm SPPs. In 2009, 1.7-µm particles with ρ = 0.73 appeared on the market and showed quite impressive kinetic performance. Minimal HETP values between 2.6 and 4.3 µm were observed with low-molecular-weight solutes, resulting in some very efficient separations of peptides (18).
The real breakthroughs in column performance came in 2013, when two providers introduced 1.3- and 1.6-µm SPP materials. The 1.3-µm packing (ρ = 0.69) provided exceptionally low minimal plate heights of ~2 µm, corresponding to a plate count of up to 500,000 plates/m (19,20). It was shown that the 1.3-µm SPP material provides the shortest analysis time among existing stationary phases for efficiencies below 30,000 plates. Despite its excellent chromatographic performance, it is evident that the efficiency of this column is limited by current instrumentation in terms of upper pressure limit and extracolumn band broadening. Even at 1000 bar, it is hard to reach optimal linear velocity, thanks to its low permeability (Kv = 1.7 × 10-11 cm2) (19). The effect of extra-column band broadening of current UHPLC equipment still has a major impact on the apparent kinetic performance when using short narrow-bore columns (50 mm × 2.1 mm) packed with a 1.3-µm SPP material. In spite of these limitations, this 1.3-µm material seems very promising for ultrafast separation of small molecules and peptides in both isocratic and gradient elution modes (19,21).
Very recently, a column packed with 1.6-µm SPPs (ρ = 0.70) also appeared on the market. The kinetic performance of 1.3-, 1.6-, and 1.7-µm materials was compared in a recent study (22). It was found that the 1.3-µm phase outperforms the two comparators in ultrafast separations. Conversely, the 1.6-µm packing seemed to be the better size for high-resolution chromatography, in isocratic and gradient modes for small molecules and peptides. This exceptional performance was attributed to its more favorable permeability (Kv = 3.5 × 10-11 cm2) and somewhat higher mechanical stability of the columns (ΔPmax of 1200 bar). Figure 2 shows the comparison of these three packings using gradient kinetic plot representation obtained from experimental data for small molecules and peptides (21). When constructing these plots, the maximum achievable peak capacity values (Pc) at any flow rate were considered for a given gradient time (tgrad) to estimate the highest achievable performance for a defined gradient time (21). For these calculations, a generic scouting gradient run from 5% B to 95% B (Δφ = 0.9) was assumed. The ratio of gradient time and achievable maximum peak capacity (tgrad/Pc) is plotted against the maximum peak capacity (Pc). In this representation, the minimal analysis time to achieve a certain peak capacity can be discussed and compared. As shown here, the 1.3-µm material is most useful for ultrafast separations requiring a peak capacity of less than 250. If higher efficiency is required, the best choice is the 1.6-µm material. The 1.6-µm material outperforms the 1.3-µm packing for small molecule separations when Pc > 300. Please note that these plots show the maximum achievable performance when operating the columns at their maximum pressure capability (kinetic performance limit).

Figure 2: Gradient kinetic plot comparison of columns packed with 1.3-, 1.6-, and 1.7-µm SPPs for the separation of (a) small molecules and (b) peptides. Maximum operating pressure: ÎPmax = 1200 bar for Cortecs and ÎPmax = 1000 bar for Kinetex columns, ÎΦ = 0.9 (5â95% B), S = 6 for butylparaben and S = 14.8 for decapeptide. Adapted from reference 22 with permission.
These sub-2-µm SPP materials were also successfully applied to the separation of tryptic digests of proteins generating 0.5–2 kDa peptides (21,22). With 50 mm × 2.1 mm columns, peak capacities between 200 and 300 were reached within 10 min (depending on the operating conditions).
Instrumental Considerations for Using Columns Packed with SPPs
Pressure Requirement: Commercially available SPP materials can be classified into three groups based on their particle sizes and operating pressure requirements: Relatively large particles of 3.6, 4, 4.6, and 5 µm; sub-3-µm particles (2.5, 2.6, and 2.7 µm); and sub-2-µm materials (1.3, 1.6, and 1.7 µm). The columns packed with 3.6–5 µm SPPs can be operated in the same pressure range as conventional 3–5 µm fully porous particles; therefore these materials can be operated on any conventional HPLC systems.
The 2.5–2.7 µm SPP packings can operate at one-half or one-third of the pressure compared to fully porous sub-2-µm particles; therefore it is theoretically possible to use these columns on conventional HPLC instruments. In generic conditions, when using an acetonitrile–water mobile phase, there is little need to go beyond 400 bar when using shorter columns packed with 2.5–2.7 µm particles. Indeed, the maximum viscosity of acetonitrile-water mixture is around 1 cP at 25 °C, while the viscosity of methanol–water and isopropanol–water mixtures can reach 1.6 and 2.9 cP at 25 °C, respectively. The nominal mechanical stability of some 2.5–2.7 µm SPP columns is 600 bar, whereas the pressure capability of conventional HPLC systems is limited to 400 bar. The pressure capability of conventional HPLC systems seems to be appropriate for columns packed with 2.5–2.7 µm SPPs, but the full benefits cannot be attained with columns of standard dimensions. Indeed, operating these columns at a pressure higher than 400 bar not only provides faster separations, but also allows for the use of alternative (more viscous) solvents to adjust selectivity.
Sub-2-µm SPP materials can be operated optimally only on UHPLC equipment, since they require operating pressures higher than 400 bar. Such columns are generally stable up to 1000–1300 bar, and their real benefits can be observed only beyond 800 bar. Because of their low permeability, very high pressure is required to operate these columns at reasonable flow rates for fast separations (19).
System Dispersion: The currently available commercial HPLC instruments can be classified into three groups according to their system dispersion: Optimized UHPLC systems for fast separations with very low dispersion (σ2ec <10 µL2); hybrid HPLC or UHPLC systems recommended by vendors for both fast and conventional separations (σ2ec = 10–50 µL2); and conventional HPLC systems with extracolumn variances over 50 µL2 (23). The extracolumn peak variances of several commercially available instruments were reported in the literature. As expected, the impact of system variance on apparent column efficiency could be critical, particularly for shorter columns in 2.1-mm i.d. format. Some model calculations were performed to illustrate the importance of system dispersion, based on experimental efficiency data observed on columns packed with SPPs of different dimensions. Figure 3 shows the effect of extracolumn variance on the percentage of remaining (observed) column efficiency for 1.3-µm (50 mm × 2.1 mm), 2.6-µm (50 mm × 2.1 mm and 50 mm × 3 mm), and 5-µm (150 mm × 4.6 mm) SPP columns (assuming an analyte with a moderate retention factor of k = 5).
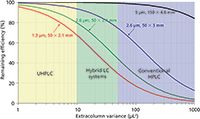
Figure 3: Effect of the extracolumn variance on the remaining (observed) column efficiency for columns packed with 1.3-, 2.6-, and 5-µm SPPs (50 mm à 2.1 mm, 50 mm à 3 mm, and 150 mm à 4.6 mm). A retention factor of k = 5 was assumed for the model calculations. The yellow area corresponds to

Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








