Developments in the Preparation of Organic Polymer Monoliths for the Separation of Small Molecules
LCGC Europe
Developments in the preparation of organic polymer monoliths suitable for the separation of small molecules in the isocratic mode are described and the main factors affecting column efficiency are identified.
Organic polymer monoliths are mainly used to separate macromolecules in gradient elution liquid chromatography (LC) because of their favourable porous structure. The main reason for the poor behaviour of organic polymer monoliths when separating small molecules isocratically was attributed to the lack of small pores with a stagnant mobile phase, and the resulting low surface area. Recent efforts have improved the separation power of organic polymer monoliths for small molecules, offering column efficiencies up to 50,000–80,000 plates/m. This review describes recent developments in the preparation of organic polymer monoliths suitable for the separation of small molecules in the isocratic mode, and discusses the main factors affecting the column efficiency.
It has been over 20 years since Hjertén published a paper describing the development and application of a "continuous polymer bed" (1). The "macroporous polymers membranes" described by Svec and Tennikova (2) followed and "continuous rods" were later introduced by Svec and Fréchet (3). These continuous materials initiated extensive research of the novel types of stationary phases that consisted of one piece of porous material filling a whole volume of a cylindrical column, later named "monolithic stationary phases" (4). Since then, monolithic stationary phases have been established as useful members of a family of stationary phases with inorganic silica-based (5) and organic polymer-based matrices (6–8).

Photo Credit: Günay Mutlu/Getty Images
The morphology of monolithic materials with an interconnected network of micrometre-sized flow-through pores enables high flow of the mobile phase at moderate back-pressures and fast separations. Silica-based monoliths are suitable for the separation of small molecules (5,9) while polymer monoliths have been successfully used for fast gradient separations of synthetic and natural polymers (10). The high temperature and chemical stability of organic polymer monoliths in the separation of low molecular compounds triggered significant efforts in tailored preparations of polymer-based, highly efficient monolithic stationary phases suitable for fast and efficient analysis of low molecular compounds. This topic has been recently reviewed by Svec (11) and Nischang (12).
This article attempts to summarize current progress in the preparation of organic polymer monoliths suitable for the separation of small molecules. Numerous protocols including adjusting the polymerization mixture composition, controlling the polymerization time and temperature, and post-polymerization surface modifications are discussed.

Polymerization Mixture Composition
Organic polymer monoliths are usually — but not always (13) — prepared by radical polymerization of monovinyl monomer and crosslinking divinyl monomer in the presence of pore forming solvents, typically alcohols (6). The polymerization reaction begins by decomposition of a radical initiator triggered by an external input, such as elevated temperature or UV light. At an early stage of the reaction, polymerization nuclei are formed and (usually) a divinyl crosslinking monomer is incorporated (7,14). At a later stage of polymerization, the nuclei grow and a highly interconnected moiety is formed. Finally, the heterogeneous, "cauliflower-like" polymeric material fills the whole fused-silica capillary space. At the end, the monolithic block is flushed to remove all unreacted components of the polymerization mixture. Figure 1 shows several examples of monomers and crosslinkers usually used for the preparation of organic polymer monoliths.
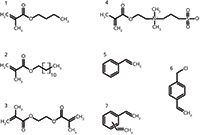
Figure 1: Examples of functional and crosslinking monomers usually used for the preparation of porous polymer monoliths. (1) butyl methacrylate, (2) lauryl methacrylate, (3) ethylene dimethacrylate, (4) N,N-dimethyl-N-methacryloxyethyl-N-(3-sulphopropyl)ammonium betaine, (5) styrene, (6) vinylbenzyl chloride, (7) divinylbenzene.
The concentration of monomers in the polymerization mixture has a significant impact on the density and efficiency of the prepared columns. Eeltink et al. improved the column efficiency from 13,000 plates/m to 67,000 plates/m by decreasing the concentration of all monomers in the polymerization mixture from 40% to 20% (15). By keeping the crosslinker concentration constant, and gradually increasing the concentration of the monomer in the polymerization mixture to 25%, the column efficiency of 30,000 plates/m was achieved at elevated temperatures (16).
Previously, the possibility of isocratic separation of low molecular alkylbenzenes has been tested on butyl methacrylate-co-ethylene dimethacrylate capillary monolithic columns (17). By increasing the 1-propanol concentration in the pore-forming mixture from 60% to 62% and then to 64% the column efficiency was improved from 5000-plates/m to 35,000 plates/m. The effect of 1-propanol on the internal structure of prepared monolithic stationary phases is demonstrated in Figure 2. At the same time, Coufal et al. used butyl methacrylate as a functional monomer and ethylene dimethacrylate as a crosslinker to prepare a monolithic column with an efficiency of 37,000 plates/m (18).

Figure 2: Effect of 1-propanol concentration on internal structure of (poly)methacrylate monolithic stationary phase. (a) 37.2% of 1-propanol in polymerization mixture, (b) 38.4% of 1-propanol in polymerization mixture. Adapted and reproduced with permission from D. Moravcová, P. Jandera, J. Urban, and J. Planeta, (2003), J. Sep. Sci. 26, 1005â1016. © John Wiley and Sons.
Different alkylmethacrylate monomers with functional groups including C2, C4, C6, C8, C12, C16, C18, and isobornyl were used to prepare monolithic stationary phases differing in hydrophobicities (16,19–22), with an efficiency in the range of 30,000–40,000 plates/m. From among the monomers, lauryl methacrylate (C12) usually showed the highest efficiency (16,23,24), which might be the result of specific interactions inside the polymerization mixture.
Horváth et al. prepared a poly(styrene-co-divinylbenzene) monolithic capillary column with a reported efficiency of 43,000 plates/m for non-retained dimethylsulphoxide (25). Another report described the efficiency of 91,000 plates/m for non-retained uracil on a poly(styrene-co-divinylbenzene) column prepared from 20% styrene, 20% divinylbenzene, 40% 1-propanol, and 20% formamide (26).
The addition of methacrylic acid into the polymerization mixture of styrene, divinylbenzene, toluene, and isooctane significantly changes the separation power of capillary columns (27). A column without methacrylic acid in the polymerization mixture showed the surface area of 0.1 m2/g and could not separate alkylbenzenes at all; however, after the addition of methacrylic acid (in the ratio of 1:1:2 of styrene, methacrylic acid, and divinylbenzene) the column had a surface area of 261 m2/g and provided a baseline separation of alkylbenzenes with the efficiency of 28,000 plates/m (Figure 3). The methacrylic acid obviously contributes to the formation of a significant number of small pores in the monolithic stationary phase (11,27).
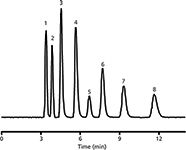
Figure 3: Examples of small molecules isocratic separations on monolithic poly(styrene-co-methacrylic acid-co-divinylbenzene) capillary column. Mobile phase: 65% aqueous acetonitrile; flow rate: 4 μL/min; column length: 170 mm; UV detection: 241 nm. Analytes: (1) thiourea, (2) phenol, (3) aniline, (4) benzene, (5) toluene, (6) ethylbenzene, (7) propylbenzene, and (8) butylbenzene. Adapted and reproduced with permission from A. Svobodová, T. KrÞek, J. Å irc, P. Šálek, E. Tesarova, P. Coufal, and K. Å tulÃk, (2011), J. Chromatogr. A 1218, 1544â1547. © Elsevier.
The addition of 2-hydroxyetlyl methacrylate in the polymerization mixture containing divinylbenzene and ethyl vinylbenzene was shown to reduce the plate height of the columns for small molecules from 160–490 μm to 40–76 μm, which was attributed mainly to the decrease in the domain size of the monoliths, leading to lower eddy dispersion and mass transfer resistance contribution to the band broadening in the column. Similar to methacrylic acid, the addition of 2-hydroxyetlyl methacrylate in the polymerization mixture enhanced the formation of small pores and significantly increased the surface area of prepared materials up to 500 m2/g (28).
The addition of polystyrene to porogen solvents was another way of increasing the efficiency of glycerol dimethacrylate-based monolithic columns for the analysis of small molecules (29). Efficiency increased from 5500 plates/m to 18,500 plates/m; and to 34,000 plates/m with the use of a low-molecular-weight polystyrene (50,000), a medium-molecular-weight polystyrene (600,000), and an ultrahigh-molecular-weight polystyrene (3,840,000) solution in chlorobenzene, respectively, in glycerol dimethacrylate-based monolith (29).
The efficiency of polymethacrylate monolithic stationary phases can also be increased by employing alkyl dimethacrylate crosslinkers with various alkyl chain lengths between the two methacrylate units (30). Hydrophobic stearyl methacrylate has been polymerized in the presence of tert-butanol and 1,4-butanediol with dimethacrylate crosslinkers including ethylene dimethacrylate, butanediol dimethacrylate, hexanediol dimethacrylate, neopentyl glycol dimethacrylate, methyl octanediol dimethacrylate, and nonanediol dimethacrylate. Even though these materials have low surface areas of only several m2/g, they allow surprisingly efficient separations of low molecular alkylbenzenes (30). Methyl octanediol dimethacrylate yielded the highest separation efficiency of 83,000 plates/m and 52,000 plates/m for unretained thiourea and retained butylbenzene, respectively.
A single monomer with crosslinking functionalities in pore-forming solvents can be used to synthesize monolithic stationary phases suitable for the separation of small molecules. Initial reports used divinylbenzene (31,32), however, it usually contains significant concentrations of styrene monomer (11). Several others monomers were used for this purpose, including glycerol dimethacrylate (29,33–36), methylene-bis-acrylamide (37), and poly(ethylene) diacrylate (38).
Tetrakis(4-vinylbenzyl)silane thermally polymerized in 1-dodecanol and toluene forms a monolithic material (39) with the surface area of 79 m2/g for 15% and 350 m2/g for 20% monomer concentration in the polymerization mixture. The column permeability decreased with higher concentration of the monomer. The monolithic columns prepared in this way were used for gradient elution separations of small molecules, including carboxylic acids, phenols, and amines. An efficiency slightly higher than 20,000 plates/m was achieved for isocratic separation of alkylbenzenes.
Lee's group prepared monolithic stationary phases using single crosslinking monomers including poly(ethylene glycol) diacrylates (40), bisphenol A dimetacrylate, bisphenol A ethoxylate diacrylate, and pentaerythritol diacrylate monostearate in cyclohexanol–decanol, methanol–diethyl ether, or mixtures of tetrahydrofuran and dimethylformamide with decanol and dodecanol as porogenic solvents. The best separation of low molecular benzene derivatives was achieved on the monolithic column based on bisphenol A dimethacrylate with dimethylformamide–dodecanol as the pore-forming solvent mixture, which showed an efficiency higher than 60,000 plates/m for butylbenzene (41).
All published reports confirm the significant impact of the polymerization mixture composition, namely its polarity at the early stages of the polymerization, when the phase separation and formation of the first crosslinked polymeric nuclei occurs. Unfortunately, there is not enough information in the current literature to allow a detailed description of the general effect of the polymerization mixture polarity on the column efficiency or permeability. The incorporation of polar methacrylate monomers in a highly nonpolar mixture of styrene and divinylbenzene (27,28) leads to the formation of materials with a high surface area that can be used for the separation of small molecules. On the other hand, low surface materials prepared from long dimethacrylate crosslinkers offer column efficiency over 50,000 plates/m for retained compounds (30). These results suggest that there might be other significant variables controlling the efficiency of the organic polymer monoliths in addition to the surface area.
The efficiency for small molecules significantly improves when substituting non-polar (poly)methylene dimethacrylates with more polar (poly)oxyethylene dimethacrylate crosslinkers, showing important effects of crosslinker polarities on the chromatographic properties of polymethacrylate monolithic columns (42). The columns prepared with tetraoxyethylene dimethacrylate crosslinker demonstrated high efficiencies of approximately 70,000 theoretical plates/m, high permeability, excellent reproducibility, and long-term stability at elevated temperatures, and can also be used in some size-exclusion applications. Figure 4 shows isocratic separation of uracil, benzene, and five lower n-alkylbenzenes on a tetraoxyethylene dimethacrylate column in 70% acetonitrile. The column showed average run-to-run errors of 0.3% in retention volumes and of 1.1% (benzene) to 3.9% (butylbenzene) in the efficiency in terms of HETP. The separation repeatability on nine columns prepared from three separate polymerization mixtures was approximately 2.5% in retention volumes and 7% in HETP for the alkylbenzene samples (42).
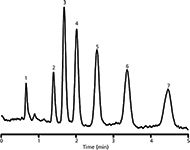
Figure 4: Separation of low-molecular alkylbenzenes on a monolithic capillary column with tetraoxyethylene dimetacrylate crosslinking monomer. Mobile phase: 70% aqueous acetonitrile; column length: 193 mm; flow rate: 17.6 μL/min; back pressure: 6.3 MPa; UV detection: 214 nm. Sample: (1) uracil, (2) benzene, (3) toluene, (4) ethylbenzene, (5) propylbenzene, (6) butylbenzene, and (7) pentylbenzene. Adapted and reproduced with permission from P. Jandera, M. Starková, V. Å kerÃková, and J. Urban, (2013), J. Chromatogr. A, 1274, 97â106. © Elsevier.
Further studies of the effects of polymerization mixture polarity and phase separation on the column efficiency will be necessary to provide new information useful for the preparation of highly efficient organic polymer monolithic columns.
Polymerization Conditions
The polymerization temperature is considered to be the variable most convenient to adjust for controlling the pore size distribution of macroporous media (43). At a higher temperature, the radical polymerization initiator decomposes faster and a significantly higher concentration of small initial nuclei is formed. Smaller voids or pores in the monolithic materials are expected if the globules are smaller; therefore a simple rule applies: The higher the temperature, the smaller the pores (43). At a higher concentration of small pores, polymer materials show a higher surface area. On the other hand, the small pores significantly decrease the column permeability, meaning that such columns are impractical for fast separations, which would require too high operating pressures.
A stable free-radical mediated preparation of porous poly(styrene-co-divinylbenzene) monoliths using a binary porogenic solvent consisting of poly(ethylene glycol) and 1-decanol at 130 °C yields an organic polymer monolith with the surface area below 200 m2/g. These materials are suitable for size-exclusion separation of organic polymers, however, they were not used in the separation of small molecules (44).
The effect of polymerization temperature on column efficiency has been studied using UV-initiated polymerization of butyl methacrylate and ethylene dimethacrylate at temperatures ranging from -15 °C to 75 °C (45). A decrease in polymerization temperature improves the column efficiency and permeability. Columns polymerized at temperatures higher than 30 °C were completely useless because of low permeability or efficiency. The highest column efficiency of 47,000 plates/m was achieved on a column prepared by polymerization at -15 °C. Another study compared the efficiency of polymethacrylate columns prepared by UV-initiated polymerization at 0 °C, 10 °C, and 20 °C from butyl methacrylate, ethylene dimethacrylate, 1-dodecanol, and cyclohexanol mixture and reported an efficiency of 45,000 plates/m on the column polymerized at 0 °C, enabling fast separation of small alkylbenzenes in seconds (46).
During the initial development of organic polymer monoliths, Svec and Fréchet studied the effect of the polymerization time on pore formation and properties of polymer materials (14). The polymer formation is the fastest at the beginning of the polymerization. At 55 °C the polymerization of glycidyl methacrylate and ethylene dimethacryalte is almost complete during the first third of the reaction time, with almost 90% polymer conversion (14). The authors compared the pore-size distribution curve of glycidyl methacrylate-co-ethylene dimethacrylate rods formed in 1 h and 22 h, respectively. The monolith formed within 1 h showed a maximum pore size distribution curve of 618 nm and a surface area of 500 m2/g, but the rod also contained a substantial amount of very large pores with diameters up to 10 μm. In contrast, pore size distribution was narrower in the material polymerized for 22 h, with a maximum of 1154 nm, and the material did not contain pores > 2 μm in diameter. The monolith after 22 h conversion contained a significant amount of small mesopores lower than 50 nm, which corresponds to the decrease in the specific surface area to 120 m2/g (14).
Nischang and Brüggemann studied the effect of the polymerization time on the performance of both poly(butyl methacrylate-co-ethylene dimethacrylate) (47) and poly(styrene-co-divinylbenzene) (48) monolithic columns. The poly(butyl methacrylate-co-ethylene dimethacrylate) monolith polymerized for 30 min provided sufficient methylene selectivity for the separation of homologeous alkylbenzenes (Figure 5). However, more retained analytes showed strong band dispersion (47). Poly(styrene-co-divinylbenzene) monolithic scaffolds polymerized for 3 h showed retention-insensitive efficiency with a plate height of 15–20 μm for low molecular alkylbenzenes (48).
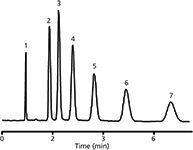
Figure 5: Isocratic separation of small molecules on monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) column obtained after a polymerization time of 30 min. Mobile phase: 50% aqueous acetonitrile; linear velocity: 3.6 mm/s. Analytes: (1) uracil, (2) benzene, (3) toluene, (4) ethylbenzene, (5) propylbenzene, (6) butylbenzene, and (7) pentylbenzene. Adapted and reproduced with permission from I. Nischang and O. Bruggemann, (2010), J. Chromatogr. A 1217, 5389â5397. © Elsevier.
Although the termination of the polymerization reaction at an early stage was reported as early as 1995 (14), it took almost 15 years before these results were used for the application of monolithic columns prepared for the separation of small molecules (49,50). Bonn's group studied the effect of the polymerization time on the properties of monolithic poly(4-methylstyrene-co-1,2-bis(4-vinylphenyl)ethane) capillary columns (50) and found an efficiency of 65,000 plates/m for the monolithic column polymerized for 45 min; the separation impaired when the time of polymerization increased. The polymerization of a single crosslinker 1,2-bis(4-vinylphenyl)ethane for 60 min allowed a monolithic column to be prepared with 72,000 plates/m for isocratic separation of phenones (49). The authors claim that decreasing the polymerization time below 60 min results in monolithic columns with both sufficient flow-channel size and a considerable fraction of small mesopores, which can be applied for a wide range of low-molecular-weight analytes on a single monolithic high performance liquid chromatography (HPLC) column (50).
Surface Modification
The independent control of the pore size distribution of both large flow-through pores and small mesopores in organic polymer monoliths is a challenge. Multivariate non-linear regression has been used to evaluate main pore formation factors in the polymethacrylate monolithic stationary phases for both flow-through pores (51) and mesopores (52). Unsurprisingly, the tailor-made monolithic columns with small pores of 30% showed significantly lower permeability than columns with very low concentrations of small pores (52). Post-polymerization hypercrosslinking modification was reported more than 20 years ago (53) but has only recently been introduced to solve the problem of the pore distribution in monolithic stationary phases (54,55).
A generic monolithic material was prepared from a mixture containing styrene and chloromethyl styrene monomers with divinylbenzene crosslinker. The pore size distribution of the monolith is controlled mainly by the phase separation at the beginning of the polymer formation. Because divinylbenzene as a bifunctional crosslinker polymerizes faster than monovinyl styrene and chloromethyl styrene monomers, the surface of the polymeric material at the end of the polymerization is covered by a layer of styrene and chloromethyl styrene polymeric chains with a very low concentration of almost consumed divinylbenzene. These layers containing chloromethyl groups are amenable to hypercrosslinking via Friedel-Crafts alkylation. The surface of the monolithic material swells in a thermodynamically good solvent such as dichloroethane; after the introduction of FeCl3 as a Friedel-Crafts catalyst and an increase in temperature, the loose polymer chains are fixed in their positions by Friedel-Crafts alkylation (54,55).
Using this protocol, organic polymer monoliths can be prepared with an independent distribution of both large flow-through pores (that originated from a generic material) and small pores on the surface of the monolith (formed by hypercrosslinking modification). The benefit of the hypercrosslinking approach is the formation of a relatively thin hypercrosslinked layer on the pore surface, while the bulk of the monolith remains impermeable to small molecules, which resembles the structure of superficially porous stationary phases (56). The significant proportion of small pores in the hypercrosslinked monolithic material dramatically increases the surface area. While the precursor monoliths only exhibited a specific surface area of 17–56 m2/g, the hypercrosslinked monoliths had surface areas of up to 631 m2/g. This value compares favourably to other polymer-based monoliths and even significantly exceeds the surface area of 300 m2/g measured for the silica monoliths (55).
Two main factors affect the efficiency of hypercrosslinked monolithic poly(styrene-co-chloromethyl styrene-co-divinylbenzene) columns in reversed-phase liquid chromatography of small molecules: Synergistic percentage of the chloromethyl styrene and antagonistic percentage of divinylbenzene crosslinker (55). As a consequence, the efficiency decreases when a higher concentration of chloromethyl styrene is present in the polymerization mixture, and, in contrast, a higher concentration of divinylbenzene provides more efficient columns. After optimization of the separation conditions, the best column produced an efficiency exceeding 80,000 plates/m for benzene under isocratic conditions (Figure 6) (55). Apart from formation of small pores, Friedel-Crafts alkylation has also been used for surface modification of prepared monoliths (26).
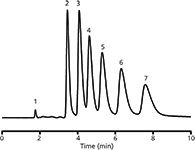
Figure 6: Separation of low-molecular alkylbenzenes on monolithic poly(styrene-co-chloromethyl styrene-co-divinylbenzene) hypercrosslinked monolithic column. Ternary mobile phase: 20% water, 20% acetonitrile, 60% THF; flow rate: 0.5 μL/min. Analytes: (1) uracil, (2) benzene, (3) toluene, (4) ethylbenzene, (5) propylbenzene, (6) butylbenzene, and (7) pentylbenzene. Adapted and reproduced with permission from J. Urban, F. Svec, and J.M.J. Fréchet, (2010), J. Chromatogr. A 1217, 8212â8221. © Elsevier.
Recently, we have used a two-step surface modification of poly(styrene-co-vinylbenzyl chloride-co-divinylbenzene) monolithic stationary phases, including hypercrosslinking and thermally initiated surface grafting of [2-(methacryloyloxy)ethyl]dimethyl(3-sulfopropyl)ammonium hydroxide, to prepare capillary columns for the isocratic separation of small polar compounds in hydrophilic interaction chromatography (HILIC). The prepared monolithic columns achieved long column lifetimes and did not lose their separation power following > 10,000 injections (57).
Svec's group has used an alternative approach for the surface modification of organic polymer monolith surfaces. They followed Horvath's pioneering work (58) incorporating carbon nanotubes in the capillary monolithic columns and used both the carbon nanotubes in the polymerization mixture, as well as the surface attachment of the cut carbon nanotubes. The optimized monolithic capillary columns with attached carbon nanotubes exhibited an efficiency of 44,000 plates/m for retained benzene (59). Very recently, the group significantly improved the efficiency of organic polymer monoliths by copolymerization of C60 fullerene-containing methacrylate monomer (60). When only a small amount (1 wt %) of the C60 fullerene-containing methacrylate monomer is included in the polymerization mixture, the morphology and column efficiency change significantly: After optimization of the polymerization and chromatographic conditions, the fullerene-incorporating monolithic columns exhibit efficiencies in excess of 110,000 plates/m for the retained benzene, which is the highest efficiency for small retained molecules achieved on organic polymer monoliths so far (60).
The same group modified hypercrosslinked high surface area monoliths with gold nanoparticles and used them as an intermediate ligand enabling the attachment of polyethyleneimine as a spacer followed by immobilization of the second layer of gold nanoparticles, which were eventually functionalized with zwitterionic cysteine. The prepared capillary columns were applied for the separation of mixtures of nucleosides and peptides in HILIC mode (61).
Analysis Conditions
As well as the presented polymerization protocols, a carefully optimized operating temperature and mobile phase composition may also significantly improve the separation efficiency of organic polymer monoliths. Because of the lower viscosity of the mobile phase and a decrease in the diffusion coefficients of the analytes, HPLC at higher temperatures is a very powerful tool for reducing the separation time. Once the operating temperature was increased from 25 °C to 55 °C, the mass transfer contribution to peak broadening on polymethacrylate monolithic columns could be decreased to approximately 50% (62). At 80 °C the separation time on the hypercrosslinked monoliths could be reduced more than twice and the efficiency increased up to 82,000 plates/m (55).
Monolithic polymethacrylate sulphobetaine zwitterionic columns stable at high temperatures have been prepared (63). An elevated temperature has a completely different effect on the retention in the reversed-phase and in the HILIC mode. While retention in the reversed-phase mode decreases at increasing temperature, because it is usual for the enthalpy-controlled retention mechanism, the retention in the HILIC mode is almost independent of temperature, suggesting primary effects of the entropic contributions to the retention. Therefore, the gain in the efficiency at elevated temperatures in the HILIC mode can be used without significantly sacrificing separation selectivity. Even though the separation efficiency at the ambient or slightly elevated temperatures was rather low, at 60–80 °C the bandwidths and resolution of phenolic compounds were generally acceptable, therefore using elevated temperatures is almost a must with the monolithic polymethacrylate zwitterionic columns. Moreover, step temperature programming combined with gradient elution can be used for fine-tuning the resolution.
By replacing acetonitrile as the organic modifier in the mobile phase with tetrahydrofuran (THF), the swelling of the polymethacrylate monolith increased and surface diffusion of solutes accelerated, leading to improved efficiency (62).
Strongly non-polar hypercrosslinked monoliths based on styrene, chloromethyl styrene, and divinylbenzene showed significant peak tailing, particularly for highly retained compounds (55). This phenomenon has already been an issue for particulate porous separation media prepared from styrene and divinylbenzene and was partly solved by using a ternary mixture comprising water and both thermodynamically poor and good solvents for the polymer (62). The addition of THF increases the strength of the mobile phase and accelerates the elution. For example, in aqueous acetonitrile amyl benzene elutes in 18 min; while with the addition of 30% THF it elutes in only 5 min (55). This approach led to a significantly improved peak shape and was attributed to the partial swelling of the polymer that fills and thus "closes" the micropores (55,64).
Conclusions
Apart from their matrix chemistry and preparation protocols, silica-based and organic polymer monoliths differ in their pore morphology. Both materials show internal structure with dominant flow-through pores but vary in the surface roughness and concentration of the small pores. While silica-based monoliths show bimodal pore structure with a significant representation of small pores (~13%) and relatively high surface area commonly reaching 300 m2/g (4,5), the pore size distribution in typical organic polymer monoliths is shifted towards dominant flow-through pores with little or no concentration of small pores and a surface area of tens of square metres per gram (55).
It was generally believed that a higher surface area of silica-based inorganic monoliths was responsible for their enhanced performance in the separation of small molecules, which was supported by the improved separation power of high surface area polymer materials, including hypercrosslinked monoliths (54,55,65) or monoliths prepared from polymerization mixtures combining the divinylbenzene crosslinker with styrene and methacrylate monomers (27,28).
Recently, Nischang and Brüggemann suggested that improved separation power of organic polymer monoliths prepared in a shorter polymerization time might be attributed to gel porosity of the polymeric material (12,47,48). The organic polymer monoliths swell in organic solvents (55,62,66,67), which causes a non-uniform layer of pores (gel porosity) absent in the dry state of the polymer. This phenomenon was studied by inverse size-exclusion chromatography by Jerabek in 1985 (66).
The gel-type porosity can be ascribed to the typical space between the molecular structural units in the polymer, resulting from the solvation and swelling, and depends on the degree of crosslinking. A swollen layer at the surface of the monolith through pores is accessible to small molecules only and contributes to enhanced mass transfer resistance (12). Differences were observed between the pore distribution of polymethacrylate monoliths measured by inverse size-exclusion chromatography in a swollen state and determined by mercury intrusion porosimetry in a dry state as shown in Table 1. As expected, the total porosity of the materials decreased with an increase in total concentration of monomers in the polymerization mixture. However, the total porosity determined in the swollen state was significantly higher than the porosity determined in the dry state, especially when determined for materials prepared with less than 30% of monomers in the polymerization mixture (67).

Table 1: Comparison of pore characteristics of the monolithic material prepared with different concentration of monomers in the polymerization mixture determined with (a) mercury-intrusion porosimetry and (b) inverse size-exclusion chromatography. Adapted and reproduced with permission from J. Urban, S. Eeltink, P. Jandera, and P.J. Schoenmakers, (2008), J. Chromatogr. A 1182, 161â168. © Elsevier.
Possible future improvements in the preparation of organic polymer-based monolithic stationary phases suitable for the fast and efficient separation of small molecules can be expected from:
(i) Better understanding of swelling properties to enable tailored preparation of organic polymer monoliths. Determination of the small pores concentration with respect to the conversion of the monolithic material will contribute to the knowledge of small pores development in the monolithic matrix during the polymerization reaction. Accurate control of the polymerization conditions, especially at the beginning of the reaction, when the reaction kinetics is at its peak will be necessary.
(ii) Optimization of the composition of the polymerization mixture with respect to its polarity affecting the swelling and phase separation in the polymerization mixture (42). The combination of polar and non-polar monomers may be helpful to increase the surface area of the monolithic material and its efficiency for isocratic separation of low molecular compounds. Exact correlation of the composition of the polymerization mixture (including pore-forming solvents) with the efficiency of a monolithic column might provide useful clues for improving column efficiency.
(iii) Optimization of the hypercrosslinking modification of the surface of monoliths to prepare a layer containing pores with suitable size distribution and thickness. Using various swelling solvents such as dichlorobutane or dichlorohexane together with other common Friedel-Crafts alkylation catalysts (SnCl4, AlCl3) may improve the column efficiency of the hypercrosslinked monoliths.
(iv) Combination of the early polymerization termination with the hypercrosslinking post-polymerization modification. Hypercrosslinking may fix the swollen polymer chains on the inner surface of the through pores in a highly crosslinked monolithic structure and could also improve the pore accessibility for the sample compounds to swollen surface.
Acknowledgement
Financial support by Grant Agency of Czech Republic projects P206/12/P049 and P206/12/0398 is gratefully acknowledged.
Jiri Urban received his PhD degree in analytical chemistry from the University of Pardubice, Czech Republic, in 2007. During his PhD, he worked in laboratories at universities in London, Eindhoven, Amsterdam, and Paris. From 2009–2011 he conducted postdoctoral research at the University of California, Berkeley, USA. Since 2011 he has worked in the Department of Analytical Chemistry at the University of Pardubice as a research scientist.
His research focuses on the preparation and characterization of organic polymer monoliths and their applications in the separation of both small and large molecules in various chromatographic modes. He is currently working with hypercrosslinked monolithic stationary phases and exploring the possibility of developing an integrated separation device.
He has published 20 scientific papers in high impact scientific journals, one book chapter, presented 19 oral presentations at international scientific conferences, and has ~500 citations with an h-index of 12. Please direct any correspondence to: Jiri.Urban@upce.cz
Pavel Jandera, Ph.D., DSc. (prof. Ing. DrSc.) is a professor of analytical chemistry in the Faculty of Chemical Technology, University of Pardubice, Czech Republic. He is vice-chairman of the Group for Chromatography and Electrophoresis of the Czech Chemical Society; member of the international committee of the Central European Group for Separation Sciences, the International Board of the "Mediterranean Separation Science Foundation Research", and the Editorial Boards of the international scientific journals Journal of Chromatography A and Analytical Letters. His research fields include high performance liquid chromatography (HPLC). theory, prediction, and optimization of HPLC separations, stationary and mobile phases in LC, programmed elution, two-dimensional LC×LC techniques, development of new stationary phases, applications in food, and environmental and industrial analysis. He has published 20 chapters in monographs, over 220 research papers, and a book. He has 5500 citations and an h-index = 41.
References
(1) S. Hjerten, J.L. Liao, and R. Zhang, J. Chromatogr. A 473, 273–275 (1989).
(2) T.B. Tennikova, B.G. Belenkii, and F. Svec, J. Liq. Chromatogr. 13, 63–70 (1990).
(3) F. Svec and J.M.J. Fréchet, Anal. Chem. 64, 820–822 (1992).
(4) G. Guiochon, J. Chromatogr. A 1168, 101–168 (2007).
(5) H. Minakuchi, K. Nakanishi, N. Soga, N. Ishizuka, and N. Tanaka, Anal. Chem. 68, 3498–3501 (1996).
(6) P. Jandera and J. Urban, J. Sep. Sci. 31, 2521–2540 (2008).
(7) F. Svec and C.G. Huber, Anal. Chem. 78, 2100–2107 (2006).
(8) M.R. Buchmeiser, Polymer 48, 2187–2198 (2007).
(9) H. Minakuchi, K. Nakanishi, N. Soga, N. Ishizuka, and N. Tanaka, J. Chromatogr. A 762, 135–146 (1997).
(10) M. Janco, D. Sykora, F. Svec, J.M.J. Fréchet, J. Schweer, and R. Holm, J. Polym. Sci. Pol. Chem. 38, 2767–2778 (2000).
(11) F. Svec, J Chromatogr A 1228, 250–262 (2012).
(12) I. Nischang, I. Teasdale, and O. Bruggemann, Anal. Bioanal. Chem. 400, 2289–2304 (2011).
(13) F. Svec, J. Chromatogr. A 1217, 902–924 (2010).
(14) F. Svec and J.M.J. Fréchet, Chem. Mat. 7, 707–715 (1995).
(15) S. Eeltink, J.M. Herrero-Martinez, G.P. Rozing, P.J. Schoenmakers, and W.T. Kok, Anal. Chem. 77, 7342–7347 (2005).
(16) Y. Ueki, T. Umemura, Y. Iwashita, T. Odake, H. Haraguchi, and K. Tsunoda, J. Chromatogr. A 1106, 106–111 (2006).
(17) D. Moravcová, P. Jandera, J. Urban, and J. Planeta, J. Sep. Sci. 26, 1005–1016 (2003).
(18) P. Coufal, M. Cihak, J. Suchankova, E. Tesarova, Z. Bosakova, and K. Stulik, J. Chromatogr. A 946, 99–106 (2002).
(19) T. Umemura, Y. Ueki, K. Tsunoda, A. Katakai, M. Tamada, and H. Haraguchi, Anal. Bioanal. Chem. 386, 566–571 (2006).
(20) X.J. Huang, Q.Q. Wang, H. Yan, Y. Huang, and B.L. Huang, J. Chromatogr. A 1062, 183–188 (2005).
(21) Z.J. Jiang, N.W. Smith, P.D. Ferguson, and M.R. Taylor, J. Biochem. Biophys. Methods 70, 39–45 (2007).
(22) B. Buszewski and M. Szumski, Chromatographia 60, S261–S267 (2004).
(23) S. Eeltink, L. Geiser, F. Svec, and J.M.J. Fréchet, J. Sep. Sci. 30, 2814–2820 (2007).
(24) J. Urban, P. Jandera, and P. Langmaier, J. Sep. Sci. 34, 2054–2062 (2011).
(25) I. Gusev, X. Huang, and C. Horvath, J. Chromatogr. A 855, 273–290 (1999).
(26) Z. Kucerova, M. Szumski, B. Buszewski, and P. Jandera, J. Sep. Sci. 30, 3018–3026 (2007).
(27) A. Svobodová, T. Krížek, J. Širc, P. Šálek, E. Tesarová, P. Coufal, and K. Štulík, J. Chromatogr. A1218, 1544–1547 (2011).
(28) K.N. Smirnov, I.A. Dyatchkov, M.V. Telnov, A.V. Pirogov, and O.A. Shpigun, J. Chromatogr. A 1218, 5010–5019 (2011).
(29) H. Aoki, T. Kubo, T. Ikegami, N. Tanaka, K. Hosoya, D. Tokuda, and N. Ishizuka, J. Chromatogr. A 1119, 66–79 (2006).
(30) Z.D. Xu, L.M. Yang, and Q.Q. Wang, J. Chromatogr. A 1216, 3098–3106 (2009).
(31) K. Kanamori, K. Nakanishi, and T. Hanada, Adv. Mater. 18, 2407–2411 (2006).
(32) J. Hasegawa, K. Kanamori, K. Nakanishi, T. Hanada, and S. Yamago, Macromolecules 42, 1270–1277 (2009).
(33) T. Kubo, N. Kimura, K. Hosoya, and K. Kaya, J. Polym. Sci. Pol. Chem. 45, 3811–3817 (2007).
(34) Y. Li, H.D. Tolley, and M.L. Lee, Anal. Chem. 81, 4406–4413 (2009).
(35) E.S. Sinitsyna, Y.N. Sergeeva, E.G. Vlakh, N.N. Saprikina, and T.B. Tennikova, React. Funct. Polym.69, 385–392 (2009).
(36) E.S. Sinitsyna, E.G. Vlakh, M.Y. Rober, and T.B. Tennikova, Polymer 52, 2132–2140 (2011).
(37) J. Hasegawa, K. Kanamori, K. Nakanishi, T. Hanada, and S. Yamago, Macromol. Rapid Commun. 30, 986–990 (2009).
(38) Z.W. Wu, K.J. Frederic, M. Talarico, and D. De Keel, Can. J. Chem. Eng. 87, 579–583 (2009).
(39) S.H. Lubbad and M.R. Buchmeiser, J. Chromatogr. A1217, 3223–3230 (2010).
(40) Y.Y. Li, H.D. Tolley, and M.L. Lee, J. Chromatogr. A 1217, 4934–4945 (2010).
(41) Y.Y. Li, H.D. Tolley, and M.L. Lee, J. Chromatogr. A 1218, 1399–1408 (2011).
(42) P. Jandera, M. Starková, V. Škeríková, and J. Urban, J. Chromatogr. A, 1274, 97–106 (2013).
(43) F. Svec and J.M.J. Fréchet, Macromolecules 28, 7580–7582 (1995).
(44) C. Viklund, A. Nordstrom, K. Irgum, F. Svec, and J.M.J. Fréchet, Macromolecules 34, 4361–4369 (2001).
(45) M. Szumski and B. Buszewski, J. Sep. Sci. 32, 2574–2581 (2009).
(46) T. Hirano, S. Kitagawa, and H. Ohtani, Anal. Sci. 25, 1107–1113 (2009).
(47) I. Nischang and O. Bruggemann, J. Chromatogr. A 1217, 5389–5397 (2010).
(48) I. Nischang, I. Teasdale, and O. Bruggemann, J. Chromatogr. A 1217, 7514–7522 (2010).
(49) A. Greiderer, L. Trojer, C.W. Huck, and G.K. Bonn, J. Chromatogr. A 1216, 7747–7754 (2009).
(50) L. Trojer, C.P. Bisjak, W. Wieder, and G.K. Bonn, J. Chromatogr. A 1216, 6303–6309 (2009).
(51) J. Urban, D. Moravcova, and P. Jandera, J. Sep. Sci. 29, 1064–1073 (2006).
(52) J. Urban, P. Jandera, and P. Schoenmakers, J. Chromatogr. A 1150, 279–289 (2007).
(53) V.A. Davankov and M.P. Tsyurupa, React. Polym. 13, 27–42 (1990).
(54) J. Urban, F. Svec, and J.M.J. Fréchet, Anal. Chem. 82, 1621–1623 (2010).
(55) J. Urban, F. Svec, and J.M.J. Fréchet, J. Chromatogr. A 1217, 8212–8221 (2010).
(56) G. Guiochon and F. Gritti, J. Chromatogr. A 1218, 1915–1938 (2011).
(57) V. Skerikova and J. Urban, J. Sep. Sci. 36, 2806–2812 (2013).
(58) Y. Li, Y. Chen, R. Xiang, D. Ciuparu, L.D. Pfefferle, C. Horváth, and J.A. Wilkins, Anal. Chem. 77, 1398–1406 (2005).
(59) S.D. Chambers, F. Svec, and J.M.J. Fréchet, J. Chromatogr. A 1218, 2546–2552 (2011).
(60) S.D. Chambers, T.W. Holcombe, F. Svec, and J.M.J. Fréchet, Anal. Chem. 83, 9478–9484 (2011).
(61) Y. Lv, Z. Lin, and F. Svec, Anal. Chem. 84, 8457–8460 (2012).
(62) Y. Huo, P.J. Schoenmakers, and W.T. Kok, J. Chromatogr. A 1175, 81–88 (2007).
(63) V. Skerikova, J. Urban, and P. Jandera, J. Chromatogr. A 1217, 7981–7989 (2010).
(64) L.D. Bowers and S. Pedigo, J. Chromatogr. 371, 243–251 (1986).
(65) T.Z. Teisseyre, J. Urban, N.W. Halpern-Manners, S.D. Chambers, V.S. Bajaj, F. Svec, and A. Pines, Anal. Chem. 83, 6004–6010 (2011).
(66) K. Jerabek, Anal. Chem. 57, 1598–1602 (1985).
(67) J. Urban, S. Eeltink, P. Jandera, and P.J. Schoenmakers, J. Chromatogr. A 1182, 161–168 (2008).


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











