Strongly Adsorbing Analytes: What, Why, and How to Fix It
In many applications of liquid chromatography (LC), excellent peak shapes are observed with simple, easy-to-use mobile phases and operating conditions. However, some specific combinations of analyte and LC column chemistry can lead to terrible peak shapes and even total adsorption of analytes to components of the LC system such that no peaks are observed at all. In this installment of “LC Troubleshooting”, I discuss two specific cases where an understanding of specific interactions between particular analyte functional groups and LC instruments or column materials is critical to achieving successful separations.
For many applications, liquid chromatography (LC) is a fantastically successful analytical technique. I often share with my students that LC instruments are found in so many laboratories because LC can be applied to many different types of problems, ranging from separation of sugars used to make biofuels to separation of therapeutic proteins. The technologies used to execute the separations—including both the instrument hardware (that is, pumps, detectors, and so on) and LC columns—have improved dramatically over the past several decades. In the case of the column itself, manufacturers are generally making columns using particles with remarkable physical and chemical stability, high purity substrates, and highly uniform morphologies. These attributes ultimately lead to high performing separations that we have come to expect, including narrow, symmetrical peaks, and excellent analyte recovery. This is what we see most of the time. However, there are problematic cases where our expectations are not met—sometimes, we observe peaks that are highly asymmetric, wider than we expect (see the December 2022 installment of LC Troubleshooting for a recent discussion of peak width expectations [1]), poorer than expected recovery, or even no recovery at all (that is, complete loss of the analyte to the system). In some cases, poor performance as measured by these characteristics can be related to strong adsorption of analytes to some component of the LC system or a component of the column itself. In this installment of “LC Troubleshooting”, I discuss specific examples of such strong adsorption, the underlying chemistry that leads to these interactions, and strategies for minimizing or eliminating the problem.
In situations where the cause of poor performance can be attributed clearly to strong adsorption of the analyte, the most robust solution to the problem is to eliminate the problem altogether. Because the analyte is the focus of our analysis, we cannot eliminate it, which means that we have to remove the other party involved in the interaction, which would be some component of the LC system or column. Sometimes, this is possible and not too inconvenient. However, in other situations, this is not possible or prohibitively expensive. Therefore, in those situations, we have to turn to managing the problem rather than eliminating it. Executing these solutions effectively requires a clear understanding of the chemical cause of the strong adsorptive interaction.
Case #1: Strong Interactions Between Strong Lewis Bases and Non-Silica Metal Oxides
Although the vast majority of LC columns in use today (especially for reversed-phase LC [RPLC]) are manufactured using particles composed of a chemically modified silica substrate, there are some applications in which particles based on other metal oxides are preferred. The most common of these include zirconia, titania, and alumina. The most attractive characteristic of these materials relative to silica for use in LC is their superior pH stability in aqueous solutions, particularly above a pH of 8. However, a practical challenge associated with these materials is that they interact strongly with analytes that have functional groups that are strong Lewis bases (for example, carboxylates). As a result, these interactions can lead to poor peak shapes unless something is done to manage the interactions.
The Chemistry Behind the Problem
Zirconium is a transition metal. In the interior of a crystal of zirconia, as in a particle used for chromatography, it typically exists in a heptacoordinate state, meaning that each zirconium atom is bonded to seven other atoms. At the surface of the particle, this is no longer possible, which means that there are d-orbitals of the zirconium that are unoccupied. These empty, electron deficient d-orbitals act as Lewis acids (electron acceptors) that interact strongly with electron-rich Lewis bases (electron donors). The Lewis bases that interact most strongly with zirconia all contain oxygen, and they are shown in Figure 1 in rank order. Blackwell and Carr have extensively and quantitatively studied the adsorption of Lewis bases on zirconia materials under chromatographic conditions (2). An LC column made with freshly prepared zirconia that has only ever been exposed to water will interact strongly with a carboxylic acid, such as benzoic acid. As a result, for all practical purposes, benzoic acid injected into the column will irreversibly adsorb to the particles. This will continue to occur until either the zirconia surface is entirely saturated with benzoic acid and there are no available surface sites for additional benzoic acid to adsorb, or a Lewis base that is stronger than carboxylate is added to the mobile phase, which will displace the carboxylates from the zirconia surface.
FIGURE 1: Interaction strength for common Lewis bases interacting with zirconia.
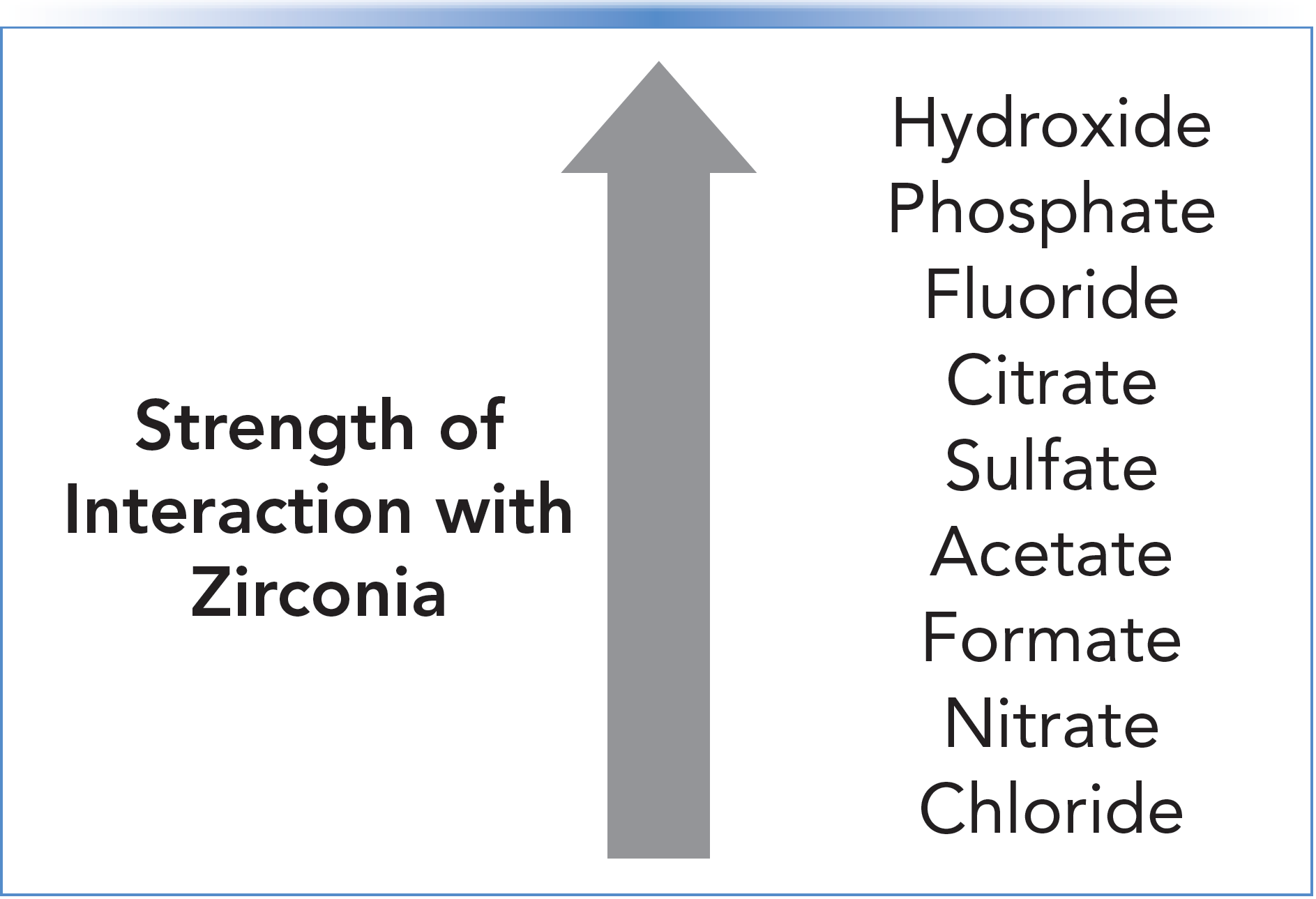
The chemistry of titania and alumina surfaces is similar to that of zirconia with respect to interactions with Lewis bases; however, they are weaker Lewis acids.
Solutions to the Problem
As discussed above, solutions to problems involving strongly adsorbing analytes fall into two broad categories—eliminating one of the partners in the interaction altogether, or managing the interaction. In the case of zirconia, titania, and alumina used as particle substrates, we cannot eliminate the interaction because there would be no particle left to work with. This leaves us to consider ways to manage the interaction, and a few different strategies have been used.
Mobile Phase Additives
The potential for negative interactions between a zirconia surface and analyte containing a functional group that is a Lewis base can be managed by adding a Lewis base to the mobile phase, specifically one that is a stronger Lewis base (higher in the rank order shown in Figure 1). In the case of benzoic acid as an analyte, adding phosphate to the mobile phase is an effective solution. In this scenario, phosphate will adsorb strongly to all available zirconia surface sites, preventing the carboxylate group of benzoic acid from adsorbing to the surface. This will dramatically improve recovery and peak shape. The downside of this solution is that the presence of the mobile phase additive may have other negative effects on the method. For example, some additives are incompatible with mass spectrometric detection (unfortunately, most of the common strong Lewis bases are MS-incompatible). Another impact of using these additives is that they impart a significant negative charge to the zirconia surface. When the zirconia is also coated with a hydrophobic polymer (for example, polybutadiene or polystyrene [3]), the addition of negative charge to the surface leads to a mixed-mode (reversed-phase and ion-exchange) retention mechanism. This can be leveraged in interesting ways to adjust selectivity for mixtures of analytes that are both charged and lipophilic enough to be retained by a reversed-phase mechanism (4). The mixed-mode character can also be challenging; for example, it can lead to very strong retention of proteins.
“Covering” Adsorption Sites
One of the great virtues of silica as a substrate for LC stationary phases is that bonding ligands to the silica surface is straightforward and versatile. Ligands with chemistries ranging from cyano to C30 can be coupled to silica through siloxane (-Si–O–Si-) bonds that are stable in aqueous solutions down to approximately a pH of 2. Analogous chemistries do not exist for modifying zirconia, titania, or alumina surfaces. As a result, commercially available stationary phases based on these substrates are typically modified by first depositing an oligomer on the surface before cross-linking those oligomers so that they are effectively permanently held in place. One might think that such coatings could effectively prevent analytes from interacting with the zirconia surface; in practice, achieving such thorough coverage has been incredibly difficult.
It Isn’t All Bad
The strong interaction between phosphate and zirconia discussed above has been used to great advantage in some specific applications. Researchers have demonstrated that this strong interaction can be used to selectively enrich phosphate-containing peptides (that is, phosphopeptides) from mixtures with other peptides (5). The peptide mixture is loaded onto a zirconia-based extraction material, followed by washing away peptides that do not contain phosphate, leaving only phosphopeptides adsorbed to the material. In a final elution step, the phosphopeptides are displaced from the material and collected for analysis, typically by LC–mass spectrometry (LC–MS).
Case #2: Strong Interactions Between Carboxylate- and Phosphate-Containing Analytes and Metal-Containing Materials
The growing importance of phosphopeptide analysis, and more recently, the characterization of therapeutic oligonucleotides that contain a phosphate-rich backbone, is making it clearer how important interactions between these molecules and metal-containing components of LC systems can be.
Chemistry Behind the Problem
Analytes with electron-rich functional groups, such as small molecule metabolites that contain carboxylates, phosphopeptides, and oligonucleotides have been shown to interact strongly with metal ions and metal-containing surfaces in LC systems. The strength of these interactions increases with the number of carboxylate, phosphate groups, or both, present in the molecule (6). Common culprits are iron and aluminum (7), although such interactions with many other metals are possible. As discussed above, in the worst-case scenario, small amounts of these analytes can be entirely lost to the LC system following injection of a sample because of complete adsorption to surfaces. In other words, no peaks are observed at the detector following injection of these analytes because they never make it that far—they get stuck somewhere in the system, and they either stay there or slowly leach out of the system over time. Everything is possible here—analytes can be adsorbed to the injector needle, valve surfaces, connecting tubing, inline filters with metal frits, LC columns made from stainless steel tubes, metal inlet and outlet frits used to hold particles inside the column, and metal ions adsorbed to the stationary phase inside the column. In other words, there are many ways for things to go wrong.
Solutions to the Problem
Eliminating or Modifying the Surfaces and Materials
In principle, it is possible to remove metals entirely from the flow path of an LC system by replacing all wetted parts with plastic or plastic-lined components. However, this significantly reduces the pressure range of the instrument in the case where all components are replaced with plastic. When plastic-lined components are used, these tend to be significantly more expensive than their stainless steel counterparts. Replacing some high surface area components is not prohibitively expensive, and definitely should be considered in this context. Two such components include solvent bottles (that is, the 1-L reservoirs that hold the mobile phase components fed to the pump), and inline filter frits. An intermediate solution that still relies on metal parts, but reduces exposure of analytes to especially problematic metals such as iron, involves the use of metal alloys that are low in iron content, or chemical modification of the metal surface (8).
Mobile Phase and Sample Additives
As in the case of zirconia and other metal oxides discussed above, mobile phase additives can be used to reduce the impact of strong adsorption of carboxylate- and phosphate-containing analytes on chromatographic performance. In this case, the addition of metal chelators to the mobile phase at low concentrations can be effective in this regard. Ethylenediaminetetraacetic acid (EDTA) is the classic metal chelator, and it is sometimes used for this purpose (7). Recently, related molecules such as medronic acid (methylenediphosphonic acid) have also been used to manage metal interactions (9). Finally, it is important to recognize that even when using a “clean” LC system with few metal parts, metals can be introduced through the injected sample, which can in turn compromise the performance of the rest of the system. In this situation, addition of EDTA to the sample itself can provide a highly effective means of sequestering free metal ions so that they do not contaminate the LC system.
Summary
In this installment of “LC Troubleshooting,” I have discussed situations where specific combinations of analyte chemistry and the characteristics of LC instrument components or columns can lead to poor peak shapes or total analyte adsorption to the LC system. Zirconia, titania, and alumina are used as alternatives to silica as a substrate for LC stationary phases for some specific applications. These materials are Lewis acids that can interact strongly with analytes containing functional groups that are strong Lewis bases. Success with this combination of analytes and LC column materials requires a mobile phase additive that is a stronger Lewis base than the functional groups present in the analytes of interest. The continuing growth in the use of LC for analysis of biomolecules such as phosphopeptides and oligonucleotides is highlighting the importance of recognizing that these molecules—which often contain multiple carboxylate or phosphate groups or both—can interact very strongly with metal-containing components of LC systems and columns. For these applications, reducing the amount of exposed metal that the analytes can interact with, using metal ion sequestering mobile phase additives, or both, is extremely important for achieving high-quality separations of these molecules.
References
(1) Stoll, D. R. Essentials of LC Troubleshooting, VI: How Wide Should Those Peaks Be? LCGC N. Am. 2022, 40 (12), 562–565,578. DOI: 10.56530/lcgc.na.cb1781m7
(2) Blackwell, J. A.; Carr, P. W. Development of an Eluotropic Series for the Chromatography of Lewis Bases on Zirconium Oxide. Anal. Chem. 1992, 64, 863–873. DOI: 10.1021/ac00032a008
(3) Zhao, J.; Carr, P. W. A Comparative Study of the Chromatographic Selectivity of Polystyrene-Coated Zirconia and Related Reversed-Phase Materials. Anal. Chem. 2000, 72 (2), 302–309. DOI: 10.1021/ac9906734
(4) Hu, Y.; Yang, X.; Carr, P. W. Mixed-Mode Reversed-Phase and Ion-Exchange Separations of Cationic Analytes on Polybutadiene-Coated Zirconia. J. Chromatogr. A 2002, 968 (1–2), 17–29. DOI: 10.1016/S0021-9673(02)00754-9
(5) Zhou, H.; Tian, R.; Ye, M.; Xu, S.; Feng, S.; Pan, C.; Jiang, X.; Li, X.; Zou, H. Highly Specific Enrichment of Phosphopeptides by Zirconium Dioxide Nanoparticles for Phosphoproteome Analysis. Electrophoresis 2007, 28 (13), 2201–2215. DOI: 10.1002/elps.200600718
(6) Nagayasu, T.; Yoshioka, C.; Imamura, K.; Nakanishi, K. Effects of Carboxyl Groups on the Adsorption Behavior of Low-Molecular-Weight Substances on a Stainless Steel Surface. J. Colloid Interface Sci. 2004, 279 (2), 296–306. DOI: 10.1016/j.jcis.2004.06.081
(7) Liu, S.; Zhang, C.; Campbell, J. L.; Zhang, H.; Yeung, K. K.-C.; Han, V. K. M.; Lajoie, G. A. Formation of Phosphopeptide-Metal Ion Complexes in Liquid Chromatography/Electrospray Mass Spectrometry and Their Influence on Phosphopeptide Detection. Rapid Commun. Mass Spectrom. 2005, 19 (19), 2747–2756. DOI: 10.1002/rcm.2105
(8) Lardeux, H.; Goyon, A.; Zhang, K.; Nguyen, J. M.; Lauber, M. A.; Guillarme, D.; D’Atri, V. The Impact of Low Adsorption Surfaces for the Analysis of DNA and RNA Oligonucleotides. J. Chromatogr. A 2022, 1677, 463324. DOI: 10.1016/j.chroma.2022.463324
(9) Hsiao, J. J.; Chu, T.-W.; Potter, O. G.; Staples, G. O.; Stoll, D., R. Troubleshooting LC Separations of Biomolecules, Part II: Passivation and Mobile-Phase Additives. LCGC N. Am. 2020, 38 (8), 431–434.
ABOUT THE COLUMN EDITOR
Dwight R. Stoll is the editor of “LC Troubleshooting.” Stoll is a professor and the co-chair of chemistry at Gustavus Adolphus College in St. Peter, Minnesota. His primary research focus is on the development of 2D-LC for both targeted and untargeted analyses. He has authored or coauthored more than 75 peer-reviewed publications and four book chapters in separation science and more than 100 conference presentations. He is also a member of LCGC’s editorial advisory board. Direct correspondence to: LCGCedit@mmhgroup.com


Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.

















