The State of the Art and Future Trends of Size-Exclusion Chromatography Packings and Columns
LCGC North America
A comprehensive review of standard and specialty SEC columns, including up-to-date charts and advice on how to choose
For this month's installment, guest authors Barth and Saunders discuss the present state of size-exclusion chromatography (SEC) columns for the separation of synthetic polymers and biopolymers. A comprehensive review of commercially available standard columns is included along with coverage of specialty columns ranging from those for small molecules to ultrahigh-molecular-weight polymers. Newer products such as low-bleed and high-throughput columns are highlighted. Column selection criteria are suggested and future directions in the technology are explored.
Recently, we provided in-depth coverage of the basics of size-exclusion chromatography (SEC) (1). In this installment, we expand on that coverage and discuss the present state of affairs with respect to SEC columns for molecular size separation of synthetic polymers and biopolymers. A comprehensive review of commercially available SEC columns from a dozen leading chromatographic companies is included ,with an emphasis on individual pore-size columns, mixed-bed packings, and wide-pore packings. Also tabulated are specialty columns for the SEC analysis of small molecules and oligomers, ultrahigh-molecular-weight polymers, water-soluble polymers, cationic polyelectrolytes, polyolefins, polar and nonpolar polymers, and carbon nanotubes. "Low-bleed" columns for use with light-scattering detectors are considered, including columns of unusual dimensions for high-throughput and rapid analysis. For those who develop SEC methods, we also include an approach for column selection based on our combined decades of experience since the inception of the method. A final section on future directions and trends of SEC column technology concludes this review.
Historical Perspective
SEC is an entropically controlled separation process (2) in which molecules in solution are separated on the basis of molecular size differences, rather than by chemical composition as with enthalpic-based separations. Size separation was first recognized as a new type of "partition" process in 1956 by Lathe and Ruthen (3,4) when they reported the separation of peptides, proteins, oligosaccharides, and polysaccharides using an aqueous mobile phase and swollen starch granules. This group correctly inferred that biopolymer "retention" was caused by their differential penetration into starch granules; larger macromolecules were eluted first because they were not able to diffuse as deeply into the granules as compared to smaller macromolecules. The extent of gel penetration or pore-volume occupancy was dictated by the molecular size or molecular weight of a sample. Soon after this discovery, gel filtration got off the ground when Pharmacia (the formerly famous Swedish pharmaceutical company) introduced a series of cross-linked dextran media, called Sephadex, with controlled porosity for the separation of biopolymers (5).
After years of failed attempts by polymer chemists, John Moore of Dow Chemical Company (6) was finally able to synthesize a series of cross-linked polystyrene resin particles of known porosities for the molecular size separation of synthetic polymers. Dow Chemical Company transferred the polymerization technology to Waters Associates, which was a small instrument company in Framingham, Massachusetts, at the time. Waters built and sold a much-needed flow-through refractometer, together with custom-made chromatographs and columns packed with cross-linked polystyrene that was synthesized in a building formerly used as a women's jail. As founder James Waters exclaimed (7), "GPC took off like a rocket!"It literally became an overnight success, like its Pharmacia counterpart.
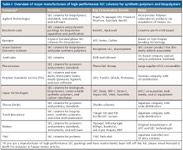
Table I: Overview of major manufacturers of high-performance SEC columns for synthetic polymers and biopolymers
Table I presents current major suppliers of SEC packings, including a brief summary of their offerings. Please note that this table does not include manufacturers of conventional large-diameter, low-efficiency packings used mainly for large-scale separations or purification, biopolymer desalting, and environmental sample cleanup. In addition to SEC products, all of these companies offer their own line of enthalpic high performance liquid chromatography (HPLC) columns, and many sell SEC chromatographs and related instrumentation.
We should recognize the early leaders and major players in the SEC arena that were the first to introduce SEC packings and through their R&D programs, helped launch and promote the technique: Waters, Polymer Labs, Pharmacia, Toyo Soda (now Tosoh Bioscience), Showa Denko, Dow Chemical Co., DuPont Co., and Synchrom Inc. See reference 8 for a list of those companies that have merged.
Entropic HPLC (SEC) vs. Enthalpic HPLC
Because of the similarities between entropic HPLC (SEC) and enthalpic HPLC, SEC closely follows in the footsteps of enthalpic HPLC in terms of packing and instrument developments. Nevertheless, there are major differences between the two modes of chromatography:
- The total SEC separation occurs within a relatively small elution volume window (that is, the total pore volume of the column), whereas with HPLC, there is no theoretical upper elution volume limit.
- Many of the polymer's characteristics (molecular weight distribution [MWD], average molecular weights, molecular size parameters, the nature of the polymerization reaction, and the sample composition) lie within the pore volume of the column (see Figure 1).
- Extracolumn peak broadening and skewing from the injector, column, detector cells, and interconnecting tubing can severely distort the polymer peak shape and compromise information within the pore volume.
- Because the diffusion coefficient and relative viscosity of a polymer sample are orders of magnitude greater than those of a small molecule, precautions must be taken to reduce their influence.
- Modern SEC with light-scattering, viscosity, and spectroscopic detectors can be used for determining molecular weights, long-chain branching, molecular size parameters, and molecular conformation all as a function of molecular weight.
- To facilitate spectral interpretation of complex spectra, SEC often is used to "clean up" complex, multicomponent samples for on-line or off-line Fourier transform infrared (FT-IR), mass spectrometry (MS), or nuclear magnetic resonance (NMR) measurements.
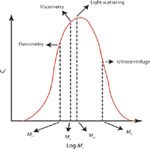
Figure 1: During an SEC analysis, the entire polymer sample is eluted within the pore volume of an SEC column. Provided that the column is calibrated with polymers of known molecular weight, the elution profile of a sample is transformed into its molecular weight distribution, from which all statistical average molecular weights can be readily determined in minutes. Before the age of SEC, these measurements would have taken many weeks to accomplish using a range of sophisticated instrumentation.
SEC Packings: State of the Art
Composition of SEC Packings
Table II lists "single-pore-size" packings, along with their particle and average pore sizes offered by major SEC companies. The polarities of mobile phases that are compatible with specific packings are designated as follows: Aq stands for aqueous (hydrophilic) mobile phases; N stands for nonpolar (hydrophobic) mobile phases; P stands for polar organic mobile phases including aqueous organic solutions; and F stands for fluorinated mobile phases. Example mobile phases for each class are given in the footnote of Table II. (When changing columns over to different mobile phases, be certain to follow manufacturer's instructions to avoid damaging the packed bed.)

Table II: Characteristics of high-performance SEC "single-pore-size" packings
There are essentially two types of SEC packing compositions: silica, with or without surface modification, and cross-linked polymeric packings, which are nonpolar (hydrophobic), hydrophilic, or ionic. The most common silica packings consist of chemically bonded 1,2-propanediol functional groups that render the surface hydrophilic. This stationary phase blocks or reacts with many of the acidic silanol groups, neutralizing the surface and making it ideal for SEC separation of biopolymers and synthetic water-soluble polymers, except for polyelectrolytes or charged polymers. Cationic polyelectrolytes or amino-containing polymers will ion exchange onto residual acidic silanol groups on bare or even diol-modified silica. Anionic polyelectrolytes or carboxylic acid–containing polymers will be ion excluded from negatively charged pores unless a significant amount of electrolyte is added to the mobile phase to shield anionic functionalities. When choosing diol-coated silica packings, especially small-pore-size packings, the pore size is reduced because of the pore volume taken up by the diol stationary phase.
Bare silica is a useful packing for the analysis of nonaqueous polar or nonpolar organic mobile phases, especially for high-temperature applications for the analysis of nonionic polymers. However, bare silica is not recommended with aqueous mobile phases because of the presence of active silanol adsorptive sites and the finite solubility of silica in aqueous buffers, especially at elevated temperatures. Furthermore, silica packings will continually degrade (that is, hydrolyze in aqueous mobile phases), exposing more silanol groups with time. A detailed summary of major SEC companies offering silica packings appears in Table II.
The newest type of silica-related packing is an ethylene-bridged hybrid inorganic-organic (BEH) packing offered by Waters Corporation (9). This packing is a mixed composition of silica and organosiloxanes which form poly-ethoxyoligosilane polymers. Compared to silica packings, BEH particles have improved chemical stability, reduced silanol activity, and a larger pore size (9).
The polymeric packing of choice for the separation of nonpolar (hydrophobic) polymers is cross-linked poly(styrene-co-divinylbenzene) (or polydivinyl benzene by Jordi Labs), introduced in 1964 by Moore (6). The popularity of this packing stems from the fact that the degree of cross-linking, which in turn influences the pore size, can be adjusted by carefully controlling polymerization conditions. Secondly, the solubility parameter of poly(styrene-co-divinylbenzene) is within the range of most vinyl types of polymers. If the packing is used with a mobile phase that has a similar solubility parameter, such as tetrahydrofuran or toluene, polymer adsorption onto the packing is prevented.
There have been a number of different hydrophilic cross-linked packings developed for the SEC of biopolymers and synthetic water-soluble polymers. Most of these packings are proprietary hydroxylated derivatives of cross-linked polymethacrylates. Unusual polymeric packings for aqueous SEC include sulfonated cross-linked polystyrene, polydivinylbenzene derivatized with glucose or anion-exchange groups, a polyamide polymer, and Novarose, a high-performance crossed-linked agarose (Table II). However, when using organic or polar SEC packings for aqueous SEC, one must guard against possible adsorption if there are extensive hydrophobic regions on the packing or polymer. Unwanted hydrophobic interactions can also occur with packings if the electrolyte concentration of the mobile phase is too high. This type of adsorption can be eliminated either by lowering the electrolyte concentration of the mobile phase, increasing column temperature, or adding an organic moderator, such as methanol, to the mobile phase (10).
Packing Pore Size
The calculated pore sizes in Table II require some explanation. Usually for rigid packings, like silica, mercury intrusion porosimetry is used to measure the average pore size, as well as the pore size distribution. However, for polymeric packings an erroneous, but historical method is applied based on the extended chain length of polystyrene. For example, all three cross-linked poly(styrene-co-divinylbenzene) packings (SDV, Polyolefin, and MCX) offered by Polymer Standards Service are available with pores as large as 1 × 107 Å or 1-mm pore size opening (see Table II). These data suggest that the pore size of a 10-µm particle is 1 mm or the pores are 100× larger than the particles themselves! The reason for this obvious impossibility is that most manufacturers still use an early proposed, but incorrect pore-size convention that is based on the extended chain length of the lowest molecular weight, excluded polystyrene standard.
This pore-size designation incorrectly assumes that a polystyrene chain length of 1 Å corresponds to a molecular weight of 40. For example, a polystyrene extended chain-length designation of 1 × 106 Å corresponds to a polystyrene molecular weight of 40 × 106 . Based on the corresponding hydrodynamic radius of a 40 × 106 MW excluded polystyrene, the estimated pore size is approximately 3000 Å, a more realistic value. Unfortunately, most column manufacturers not only use this extended-chain designation, but to complicate matters, fail to inform users. In Table II, we have tried to identify those packings that still rely on this antiquated designation. Regardless of the pore-size designations that are used, these extremely large polymer molecular weight exclusion limits offered by suppliers are beyond the practical range of polymer separations, unless one is using these packings for the separation of nanosize particles or associated molecular structures.
Coupled Columns, Mixed-Bed Columns, and Wide-Pore Packings
There are three methods available for extending the molecular weight separation range and improving the linearity of the SEC calibration slope. The first method calls for coupling together columns that have adjacent molecular weight separation ranges but the same calibration slopes, or matched V i /V0 ratios, where V i is the pore volume and V o is the interstitial volume. The second approach, which has been used quite successfully, is to mix together (in the same column) calculated fractions of two or three single-pore packings of slightly overlapping molecular weight ranges but with the same pore volume or V i /V0 ratio. These "mixed-bed" or "linear columns" have linear and shallow calibration slopes, as presented in Table III. These columns are very useful for laboratories that receive a variety of polymer samples of unknown and sometimes broad distributions. Nevertheless, small calibration slope discontinuities or deviations from linearity can still occur.

Table II: Continued
The third type of column packing, which was met with rave reviews, comprises the so-called "multipore" or "wide-pore" packings that are listed in Table III. These packings consist of particles that contain multiple or a broad distribution of pore sizes. Calibration plots of the three molecular weight ranges of multipore columns manufactured by Tosoh Bioscience are shown in Figure 2. TSKgel SuperMultiporeHZ-M (4 µm) and SuperMultiporeHZ-H (6 µm) give extraordinarily linear calibration plots with no observable calibration slope discontinuities. SuperMultiporeHZ-H has a linear molecular weight range from small molecules (1 × 102 ) to ultrahigh-molecular-weight polymers (1 × 107 ) with respect to polystyrene: a five-order molecular weight range for a single 15-cm column with a separation time of less than 6.5 min. The separation range for the -M column is about an order of magnitude lower, but the calibration slope is much lower, which is a desired feature. The molecular weight separation range of the -N column is only three-orders of magnitude, 1 × 102 to 1 × 105 , but the slope is remarkably the lowest of the three columns. Unfortunately this column has a second, steeper region from 1 × 105 to 5 × 106 that will distort the shape of the high-molecular-weight end of the chromatogram tracing. Please note that Tosoh Bioscience recently has introduced wide-pore-size packings, the TSKgel SuperMultipore PW series, for SEC analysis of water-soluble polymers. Agilent Technologies and BioChrom Labs also market their versions of wide-pore SEC packings, as listed in the third row of Table III.

Table III: Characteristics of specialty high-performance SEC packings*
Distorted Peak Profiles
Modern SEC columns now have unprecedented column efficiencies, approaching >130,000 plates/m for 3-µm packings using a small-molecule test solute. With high-efficiency and large-pore-volume packings, we are now able to detect previously unresolved components not seen with older column technology. High-efficiency columns are more sensitive to MWD distortions that arise from nonlinear regions of the calibration plot. An example of the latter is seen in Figure 3 which shows a series of SEC chromatograms of four different lots of a phenolic resin. These phenolic resins have wide MWDs, especially lot D. Starting at 8 min (2.8-mL elution volume), individual oligomers appear up to the negative solvent peak at 10 min.
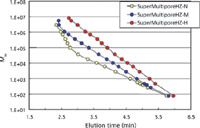
Figure 2: Calibration curves for individual TSKgel SuperMultiporeHZ-N (3 µm) â, SuperMultiporeHZ-M (4 µm) â, SuperMultiporeHZ-H (6 µm) â. Column dimensions: 150 mm Ã4.6 mm; mobile phase: tetrahydrofuran; flow rate: 0.35 mL/min; detection: UV absorbance at 254 nm; temperature: 25 °C; samples: standard polystyrene kit (PStQuick kit-H) with benzene as solvent; injection volume: 5 µL. Courtesy of Tosoh Bioscience; adapted from reference 11.
An unusual feature of four of these chromatograms is the distorted profile in lots B, C, and D occurring at the same 6-min elution volume. Because these phenolic resins are produced using condensation polymerization, we would not expect perturbation in the higher-molecular-weight region, unless, of course, the polymerization was altered during production, or a second phenol resin was added to the original lot — unlikely events. It has been suggested by Tosoh Bioscience researchers (11) that this distortion, not normally seen with lower-resolution columns, is caused by column–column inconsistencies when two differently packed columns are coupled together; in Figure 3, a TSKgel SuperHZ-3000 column is added to a SuperHZ-2000 column. Although both columns have the same dimensions, properties of the columns, like different pore volumes and severely overlapping molecular weight ranges, can give rise to distorted, nonlinear slopes. (Please note that it may be possible to compensate for calibration nonlinearity by defining the calibration curve with a sufficient number of polymer standards, especially in the region where the inflection point occurs.)
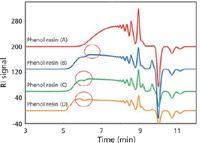
Figure 3: Composite SEC chromatograms of four phenolic resin samples analyzed with a two-column set of "single-pore size" packings: TSKgel SuperHZ 3000 + 2000 (150 mm Ã4.6 mm) Ã2; mobile phase: tetrahydrofuran; flow rate: 0.35 mL/min; detector: UV absorbance at 254 nm; temperature: 25 °C; samples: phenolic resins (3 µg/µL); injection volume: 10 µL. Courtesy of Tosoh Bioscience; adapted from reference 11.
If the same phenolic resins are analyzed this time with two identical TSKgel SuperMultipore HZ-N columns, which have the same dimensions and the same particle size (3 µm) as those used to produce Figure 3 chromatograms, we arrive at the expected profiles in Figure 4 (11): The distortions at the beginning of the chromatograms are absent. We must stress, however, that if the proper molecular weight calibration plot is constructed with the SuperHZ3000 + 2000 set using a sufficient number of standards and a nonlinear fit, both column sets shown in Figures 3 and 4 should give identical MWDs for all of the phenolic resins, without MWD distortion.
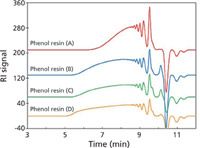
Figure 4: Composite SEC chromatograms of four different phenolic resins analyzed with a two-column set of "multipore" (wide-pore) packings: TSKgel SuperMultiporeHZ-N (150 mm Ã4.6 mm) Ã2; see Figure 3 for all other chromatographic conditions. Courtesy of Tosoh Bioscience; adapted from reference 11.
The final example of chromatogram distortion caused by nonlinear calibration behavior is given in Figure 5 for the SEC analysis of a different batch of phenolic resin. When a set of four single-pore TSKgel SuperHZ columns was used, considerable distortion was obtained, ostensibly caused by considerable molecular weight overlapping among columns that also have different calibration curve slopes. With the use of a truly linear SuperMultiporeHZ-M four-column set, distortion disappears. If one were not aware of this anomalous, but not infrequently occurring effect, it would have appeared that this phenolic resin had experienced a dramatic upset during polymerization, and the entire lot of phenolic resin could have been lost at great expense.
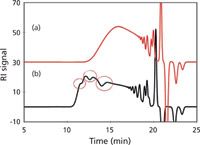
Figure 5: Composite SEC chromatograms of a phenolic resin sample analyzed with a four-column set of (a) "multipore" (wide-pore) packings: TSKgel SuperMultiporeHZ-M (150 mm Ã4.6 mm) Ã4; (b) "single-pore size" packings: TSKgel SuperHZ4000 + 3000 + 2500 + 2000 (150 mm Ã4.6 mm) Ã4; see Figure 3 for all other chromatographic conditions. Courtesy of Tosoh Bioscience; adapted from reference 11.
Specialty SEC Columns
We can assume that the lowest-particle-size packing within each designation in Table II will produce the most efficient columns. In addition, the smallest- and largest-pore-size packings will be especially useful for small molecules and oligomers, and for ultrahigh-molecular-weight polymers, respectively. For a given sample molecular weight range and chemical composition, this table should prove useful for selecting appropriate single-pore columns or for putting together a useful column set.
Available column dimensions are not included in these tables, because most column sizes are readily obtainable or can be custom packed. Column suppliers, in general, offer a broad range of column lengths and inner diameters for high-speed and high-throughput SEC separations, including large-internal-diameter columns (>20 mm) for semipreparative and preparative separations.
Many specialty SEC columns are now available from vendors, as listed in Tables III and IV. Columns capable of operating at 220 °C are now available from Agilent; silica packings also can be used at elevated temperatures because of their innate stability. Several suppliers now have packings that are custom polymerized to prevent particle fines from either forming or sloughing off the column, giving spurious spikes if light-scattering detection is used. Columns can also be conditioned for light-scattering applications by extensive elution with the mobile phase of choice.
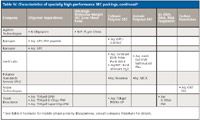
Table IV: Characteristics of specialty high-performance SEC packings, continued*
There are some interesting packings termed universal or multimode that can be used for SEC, as well as for enthalpic HPLC. In principle, one can first analyze a sample using SEC by eluting with, for example, a nonpolar mobile phase under isocratic conditions. For the second stage of analysis, gradient elution might be used with a different set of mobile phases to elute and separate adsorbed components left on the column from the SEC run.
Polymer Standards Service has an interesting asymmetric column configuration that has dimensions of 5 cm × 2 cm i.d. for high efficiency separations of only a few minutes (see Figure 6).
Lastly, as listed in Table IV, specialty SEC columns have been developed for determining 1. ultrahigh-molecular-weight synthetic polymers under low-shear-rate conditions to avoid polymer shear degradation; 2. the MWD of cationic and anionic polyelectrolytes; 3. the MWD of ds-DNA and high-molecular-weight RNA and DNA fragments; and 4. the size separation of DNA-wrapped carbon nanotubes.
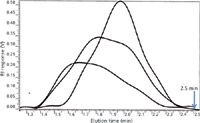
Figure 6: High-temperature, high-speed, high-flow rate SEC of polyethylene samples in under 3 min. A 50 mm Ã20 mm polyolefin mixed-bed column (10 µm) was used at a flow rate of 5 mL/min, with trichlorobenzene as the mobile phase at 145 °C with a Waters 150 chromatograph. Adapted from Polymer Standards Service GmbH, Germany.
Column Selection
Column selection for SEC is usually a straightforward task. After choosing an appropriate SEC-column vendor, one next picks a packing that is compatible with the mobile phase required, and a pore size that will cover the anticipated molecular weight range of samples, using Table II for guidance. If the expected molecular weight range is narrow, that is, less than two orders of magnitude, it is best to choose a single-pore column; if the molecular weight spread is broad, that is, greater than two orders of magnitude, a mixed-bed or wide-pore column should be selected.
The best way of choosing packing pore size is by comparing SEC calibration plots that are posted for each column type. The appropriate column or column set can be chosen from the molecular weight range, the slope linearity in which smooth slopes without inflections or discontinuities are desired, and the slope steepness, whereby shallow slopes give the best resolution. Please remember, however, that these calibration plot characteristics supplied by the manufacturer depend on the chemical composition of the polymer and the nature of the mobile phase used to construct the plot.
If you find that you can tailor-make a column set with superior characteristics compared to purchasing either mixed bed or wide-pore size columns, the following guidelines should be used:
- The inner diameters of all columns in the set should be the same.
- The type of packing in each column should be matched.
- The slopes of all of the SEC calibration plots should be the same.
- All columns should have the same pore volume or pore volume to interstitial volume ratio.
- SEC calibration plots should be adjacent to one another, but not overlapping (12).
- The pore-size order of the column set is inconsequential (13).
After the correct pore size is selected, a column with the smallest particle size, or highest efficiency should be chosen. It is curious that many SEC column manufacturers tend to keep an extensive backlog of less-efficient high-performance columns, even though they have more highly efficient columns listed. If there is a choice, users should always purchase the state-of-the-art, highest efficiency column that meets their needs. Even a small increase in column efficiency or resolution may uncover hidden components that may otherwise go undetected, or reveal that product failure was caused by a slight polydispersity increase. More importantly, however, is the fact that accuracy of molecular weight measurements increases somewhat exponentially with column efficiency (12).
As we have previously discussed (1), any HPLC column in principle can be used for SEC by adjusting the mobile-phase composition so that there are no enthalpic interactions between packing and sample. Pore size and pore-size distribution for a given single-pore column should be uniform and tightly controlled, and available in at least three or four pore sizes to cover a broad molecular weight range from oligomers to ultrahigh-molecular-weight components of about 106 . Because high-molecular-weight solutes have diffusion coefficients orders of magnitude smaller than those normally encountered in HPLC, it is critical that the smallest SEC particles be used.
Although column pore-size listings are quite useful for selecting columns of the correct molecular weight range, we feel that it is more instructive to consult actual SEC calibration plots of a number of test polymers to choose columns that give shallow and smooth linear slopes. Consequently, we recommend that users study product literature before making a selection of columns. However, bear in mind that the slope and log MW and elution volume intercepts depend on the molecular conformation and monomer molecular weight of the polymer under study. When comparing columns, choose the ones that have the highest resolution — that is, the lowest amount of broadening and the lowest calibration slope according to the following equation (12):
R*sp = 0.58/σ'D2L½ [1]
where σ' is the amount of peak broadening, that is, standard deviation in units of volume of a small monodisperse solute; D2 is the negative slope of the SEC calibration plot (log MW vs. elution volume); and R*sp is the specific resolution normalized with respect to column length L.
Many first-time SEC users assume incorrectly that a single-pore-size packing is only capable of separating or retaining macromolecules whose molecular size matches the pore openings of a packing. However, size separation does not depend on whether or not a macromolecule can penetrate or occupy a pore; rather, it depends on the fraction of pore volume a macromolecule can occupy, provided that it is sufficiently small to penetrate the pores in the first place. This pore-volume fraction is actually defined by the SEC distribution coefficient KSEC :
KSEC = (VR - V0)/V i [2]
where VR is the elution volume of a polymer, V0 is the interstitial volume of the packed column, and V i is the total pore volume of the packed column. Because a single-pore-size packing can accommodate a wide molecular-size or molecular-weight range (12), the slope of the SEC calibration plot of log M vs. VR, is finite, rather than infinite or perpendicular to the abscissa. Except for some tailor-made porous materials, silica and polymeric SEC packings have finite pore-size and pore-opening distributions, as well as different pore shapes, further decreasing the SEC calibration curve slope (12).
Column Purchasing Caveats
SEC users should also keep in mind that some SEC packing material will slowly degrade with time. In the case of polymeric packings stored in nonstabilized tetrahydrofuran, peroxide buildup will degrade the packing over time. Even if stabilized solvents are used for column storage (that is, solvents that contain an antioxidant such as BHT), oxidized products might adsorb onto the packing, changing its adsorptive properties. From extensive studies involving silica packings, long-term storage in aqueous solutions, especially if neutral or slightly basic, can degrade silica, thereby lowering column efficiency and generating fines that will eventually interfere with on-line light scattering detection. In view of these potential problems, buyers should insist on purchasing newly packed columns. Although some manufacturers supply a dated test chromatogram with each column sold, it would be more desirable for all column producers to list the manufacturing date, or specifically the date the column was packed. This information can sometimes be traced if the column serial number is provided.
Our last caveat involving column selection is about the risk of purchasing packings that may contain inadvertently adsorbed components on the surface. Depending on how the packing is polymerized, packed into columns, and washed, it may contain any number of adsorbed components that could possibly change the adsorptive properties of the polymer packing. Packings can also adsorb impurities from mobile phases or from injected samples overtime. In light of this potential problem, we recommend that SEC columns in question be evaluated by injecting polymer samples of interest and measuring column recovery, which should be greater than 95%. Columns also should be tested for unwanted adsorption by injecting the monomer or dimer forms of the sample in question, and measuring elution volume or SEC distribution coefficient, KSEC (equation 2). The KSEC values should be close to unity to ensure the absence of adsorption. If adsorption is suspected, higher-molecular-weight samples will probably be retained on the column, that is, KSEC > 1, invalidating the SEC process. In the likelihood of adsorption, columns should be cleaned of adsorbed components by following the manufacturer's recommendations, or another column brand should be tested.
Anticipated and Recommended Future Trends
SEC is a well-established technique for routine polymer and biopolymer separations, common to most laboratories that deal with macromolecules. As such, developments in column technology have matured and slowed in recent years because many of the parameters that affect the performance of a size-exclusion separation have been researched and optimized.
SEC separations rely heavily on the physical properties of packings, as summarized earlier (1). Thus, in this section we focus on these inherent properties that can only be adjusted and controlled through manufacturing.
Pore Volume
The most important packing properties are the pore size, which define the molecular weight separation range, and the pore volume, V i , or the ratio V i /V o , which helps to establish the resolution of a separation. It appears that we are close to the maximum limit of Vi/V o of commercially available cross-linked polymer packings; to obtain a significantly higher V i /V o ratio, a paradigm shift in column technology is required. An open network of structurally sound pores needs to be developed; perhaps a new type of silicate needs to be realized, as with Waters BEH column technology (9).
To substantially increase V i , large-diameter columns can be used, a course taken by Polymer Standards Service's asymmetric column. An expensive proposition is to use preparative SEC columns for analytical work. Experimentally, Vi can be increased by simply adding more columns in series. In both of these cases, however, analysis time is compromised unless higher flow rates are used.
Peak Broadening
For most SEC applications involving polydisperse macromolecules using high-efficiency columns and standard calibration methods, the relative molecular weight accuracy caused by column peak broadening is about 1%, which is quite acceptable considering other sources of extracolumn peak broadening. Some of these other sources of error, such as injection volume, can be easily reduced. Furthermore, broadening and skewing corrections can be applied to further increase molecular weight measurement accuracy. If greater resolution is required for the SEC analysis of small molecules and oligomers, enthalpic separations should be considered because of its higher peak capacity. Furthermore, for monodisperse biopolymers, MS is the obvious choice.
We have been suggesting for a number of years that a combination detector cell would certainly be advantageous and a major step forward in the advancement of SEC. We envision a combination detector in which a single low-volume cell is used for four simultaneous measurements: 1. light scattering (integral or dynamic) for molecular weight and size measurements; 2. refractive index as a concentration detector; 3. UV absorbance for either concentration or for chemical heterogeneity; and 4. FT-IR for short-chain branching or chemical heterogeneity. One possibility would be to use a charge-coupled device for multiangle and multiwavelength light scattering detection. The elimination of interdetector volume would greatly reduce peak broadening and skewing.
Particle Size
Decreasing the particle size of packings, of course, is fundamental to high-performance separations. Although polymers with large hydrodynamic volumes and elongated conformation will fragment at high linear velocity, as discussed in some detail earlier (1,14), we must realize that most polymers of commercial interest fall below the roughly 1 × 106 limiting molecular weight value. Regardless, at least one column vendor has addressed this issue by supplying columns of larger particle size and larger frit sizes, specifically for the analysis of large macromolecules (see Table IV).
Pore Size
The pore size of packings controls the molecular weight operating range over which a separation is achieved. Some packings are able to separate molecules ranging from monomers to an estimated (that is, extrapolated) molecular weight value of up to ~107 or ~108 depending on the standards used. (Note that compact polymers, like branched polysaccharides, synthetic dendrimers, and globular proteins have much higher exclusion limits.) This range obviously covers all possible applications, so it is unlikely that there will be any major developments in this area. For single-pore-size packings (Table II), a tight pore-size distribution would be very beneficial, provided that a range of pore sizes are available that have similar pore volumes for a given packing type.
Reduced-Diameter Columns and Small-Bore Columns
Low-dispersion HPLC instrumentation and MS require the use of narrow-bore SEC columns, rather than the standard analytical internal diameters of 6–8 mm. However, total pore volume becomes critically small and extracolumn peak broadening will severely limit applicability of small bore-size SEC columns. The future trend involving SEC column internal diameter actually is in the opposite direction; large internal diameter columns offer more advantages.
SEC and HDC
There have been studies on the use of SEC columns for performing both size exclusion and hydrodynamic chromatography (HDC) in a single run (15). In HDC, excluded macromolecules are separated within the interstitial volume of the packed bed by sampling different velocity streamlines. For example, larger macromolecules, on average, spend more time in the faster velocity streamlines than smaller-size macromolecules do. Smaller macromolecules can get closer to the walls of adjacent particles and can sample, on average, slower moving stream lines. The net result is that high-molecular-weight components are eluted first, followed by smaller-sized chains; the same elution order as SEC.
Although the dual separation mechanism is an interesting concept, HDC suffers from two of the same limitations as SEC does: HDC has a small elution volume range, in effect, the interstitial volume is close to the pore volume. Furthermore, macromolecules are exposed to the same elongational strain rates as in SEC; however, elongational strain at pore openings is absent with HDC. As a result, there is probably less shear rate degradation of macromolecules during HDC compared to SEC (14). Nevertheless, the single benefit HDC has over SEC is its lower peak broadening caused by the absence of nonequilibrium effects, that is, elimination of mass transfer of macromolecules into and out of pores, especially if nonporous particles are used for HDC. It is important to realize that HDC of higher-molecular-weight macromolecules is possible, only if extremely low linear velocities are used.
It is of interest to realize that the HDC mechanism is probably present to some extent in all SEC separations, but probably goes unnoticed since polymer peaks elute in the same order as with SEC. Dedicated SEC–HDC columns have yet to be commercialized for polymer separations, although specialized columns consisting of larger-diameter packings for particle-size analysis are available.
Nonporous Packings
Although pellicular particles offer improved flow properties and appear to have higher efficiency than porous SEC packings, their limited interstitial volume places more emphasis on using SEC systems with exceptionally low extracolumn volume. Furthermore, they have rather steep calibration slopes, thus they seem to be of limited interest unless used in the HDC mode.
Monolithic Columns
Monolithic columns have received more attention recently, but currently lack pores of the correct dimensions for size separations that would compete with traditional SEC columns (16). It is anticipated that work will continue in this area, but results may not be of commercial relevance.
High-Throughput Analysis
The usual approach for rapid analysis is to use short or narrow-bore columns, preferably packed with small particles for increased efficiency. However, the difficulty inherent in using these approaches for SEC is pore-volume reduction, which will magnify errors caused by extracolumn volume, and flow-rate and temperature variability, all of which will affect baseline drift and detector noise. Considering these difficulties, SEC instrumentation has been introduced that has exceptional temperature control and flow-rate stability, as in the case of Tosoh Bioscience's recently introduced EcoSEC GPC system (17) and Agilent's PL-GPC 220 system for dedicated SEC operation at high temperature.
Still another way of performing high-speed SEC separations, put forward by Polymer Standards Service, is to use large-diameter, short columns operated at high flow rates. These short and fat asymmetric columns have excellent resolution because of their large pore volume.
Related to high-throughput separations for the analytical laboratory setting is the need for on-line, at-line, or off-line SEC instrumentation, specifically designed for process control of polymerizations or quality assurance of polymers, which appears to have taken a back seat. There is no reason why SEC instrumentation, equipped with all necessary safety features, should not be an integral part of plant control laboratories involved with polymer production or biopolymer isolation and purification.
Packing Composition
One of the more vexing problems encountered in SEC used to be the limited availability of packings for the analysis of water-soluble polymers, specifically polyelectrolytes and especially cationic polymers that are very adsorbent. As shown in Table IV, there are now a number of packings available for these types of polymers.
Even though it appears that advancements in SEC column development have leveled off, there is always room for improvement, especially for stable, high-porosity structures with sufficient pore volume. The new types of hybrid packings introduced by Waters (9), for example, appear to be a promising approach for the development of new packings.
Summary
Given the maturity of SEC column technology and that the fundamental parameters affecting column resolution and performance have been well studied over the years, most advances are likely to take the form of minor modifications and improvements to satisfy specific needs and applications, such as alterations in surface chemistry of packings or reduced column dimensions to allow coupling to MS. Nevertheless, the development of smaller particle sizes continues and could lead to columns with marked resolution improvements over existing technologies. Research in the area of hybrid silica and organosiloxane particle technology appears very encouraging, especially since these particles have improved chemical stability, reduced silanol activity, and larger pore sizes compared with silica particles. Finally, considering the interest in high-pressure HPLC systems, this is seen as the most significant and likely route for future SEC column development.
Because our goal as analytical chemists is to provide MWD data of samples, perhaps we should not forget to include other nonchromatographic means of high-resolution separations, especially for high-molecular-weight macromolecules: thermal and flow field-flow fractionation, modern ultracentrifugation, and matrix-assisted laser-desorption ionization time-of-flight MS. In addition to these techniques, the molecular weights of biopolymers, especially proteins, are being characterized using one- or two-dimensional polyacrylamide gel electrophoresis and capillary zone electrophoresis, all of which represent active areas of innovation and research (18). Suffice it to say, these areas have their limitations but offer some interesting advantages over SEC.
Acknowledgments
Both authors are indebted to Dr. Ronald E. Majors of Agilent Technologies, Inc., for his help and guidance in the preparation of this manuscript. One of the authors (H.G.B.) expresses his gratitude to Cara Tomasek, Tosoh Bioscience, for supplying some of the figures for publication.
Howard G. Barth is now retired from the DuPont Company, where he was senior research associate. He is available for consultation in chromatography, analytical chemistry, and technical editing and writing. Please direct correspondence to: howardbarth@gmail.com.

Howard G. Barth
Greg D. Saunders is the product manager responsible for the GPC/SEC consumables portfolio at Agilent Technologies.

Greg D. Saunders
Ronald E. Majors "Column Watch" Editor Ronald E. Majors is a Senior Scientist in the Columns and Supplies Division at Agilent Technologies (Wilmington, Delaware), and is a member of LCGC's editorial advisory board. Direct correspondence about this column to lcgcedit@lcgcmag.com.

Ronald E. Majors
References
(1) H.G. Barth and G.D. Saunders, LCGC No. America, Recent Developments in LC Column Technology 30(S4), 46 (2012).
(2) Different names have been used to denote entropically controlled HPLC, as compared to enthalpically controlled HPLC: commonly used terms are size-exclusion chromatography (SEC), gel filtration chromatography (GFC), gel permeation chromatography (GPC), and the latest is entropic HPLC (E-HPLC). In this article, SEC is used exclusively as the generic name, while GPC and GFC are reserved specifically for the separation of macromolecules using organic or aqueous mobile phases, respectively. Because the term "filtration" is misleading, we recommend against the use of the term GFC.
(3) G.H. Lathe and C.R.J. Ruthven, Biochem. J. 62, 665 (1956).
(4) H. Determann, Gel Chromatography, 2nd Ed. (Springer verlag, New York, New York, 1969).
(5) J. Porath and P. Flodin, Nature 183, 1657 (1959).
(6) J.C. Moore, J. Polym. Sci. A2, 835 (1964).
(7) L.S. Ettre, LCGC No. America 23(8), 752 (2005).
(8) Synchrom Inc. first merged with Micra, which in turn merged with Eprogen; DuPont Zorbax technology, Varian Inc., and Polymer Labs are now part of Agilent; Pharmacia is now part of GE Health Care.
(9) K. Wydham, T. Walter, P. Iraneta, B. Alden, E. Bouvier, C. Hudallah, N. Lawrence, and D. Walsh, LCGC No. America, Recent Developments in LC Column Technology 30(S4), 20 (2012).
(10) S. Mori and H.G. Barth, Size Exclusion Chromatography (Springer Verlag, Berlin, Germany, 1999).
(11) Tosoh Bioscience, Product Guide: TSKgel HPLC Columns, 59 (2011).
(12) A.M. Striegel, W.W. Yau, J.J. Kirkland, and D.D. Bly, Modern Size Exclusion Chromatography, 2nd Ed. (Wiley, New York, New York, 1999).
(13) B.Kempf, R. Eksteen, and H.G. Barth, LCGC No. America 29(8), 668 (2011).
(14) H.G. Barth and F.C. Carlin, J. Liq. Chromatogr. 7, 1717 (1984).
(15) S.S. Huang, in Handbook of Size Exclusion Chromatography and Related Techniques, 2nd Ed., C.-S. Wu, Ed. (M. Dekker, New York, New York, 2004), p. 677.
(16) K. Cabera, LCGC No. America, Recent Developments in LC Column Technology 30(S4), 30 (2012).
(17) X. Villarreal and H.G. Barth, Am. Lab. 41(4), (2009).
(18) D.A. Egas and M.J. Wirth, Annu. Rev. Anal. Chem. 1, 833 (2008).

The 2025 Lifetime Achievement and Emerging Leader in Chromatography Awards
February 11th 2025Christopher A. Pohl and Katelynn A. Perrault Uptmor are the winners of the 18th annual LCGC Lifetime Achievement and Emerging Leader in Chromatography Awards, respectively. The LCGC Awards honor the work of talented separation scientists at different stages in their career (See Table I, accessible through the QR code at the end of the article). The award winners will be honored during an oral symposium at the Pittcon 2025 conference held March 1-5, in Boston, Massachusetts.
USP CEO Discusses Quality and Partnership in Pharma
December 11th 2024Ronald Piervincenzi, chief executive officer of the United States Pharmacoepia, focused on how collaboration and component quality can improve worldwide pharmaceutical production standards during a lecture at the Eastern Analytical Symposium (EAS) last month.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





