50 Years in Chromatography and Sorption Techniques: A Look from the East
LCGC North America
In both East and West, new ideas have often met resistance.
Even on the Eastern side of the Iron Curtain, chromatography was advancing in the latter half of the 20th century. But in both the West and the East, substantially new ideas have always needed time to become established in the minds of scientists and in industry practice.
Probably no one — at least no one in the separation science community — would dispute the statement that chromatography has played the role of "the science of sciences" in the past century, by changing, in a most revolutionary manner, research techniques and the potential of all branches of natural sciences. And yet, after a full century of combined efforts of thousands of chromatography enthusiasts, this technique continues to be an exciting and apparently inexhaustible field of research. Of course, the history of chromatography is the superimposition of contributions of many successful researchers, some operating under favorable conditions, and others, on the opposite end of the scale, struggling against an endless chain of life problems and against less-creative but highly positioned contemporaries. Interestingly, the most revolutionary innovations are often met with disapproval and resistance by the scientific community, particularly when the new ideas contradict the generally accepted opinions formulated by a few "indisputable" authorities.
From this point of view, most tragic seems to be the fate of Michael Tswett, the creator of chromatography (1). Although he was born in Italy (in 1872) and received a doctoral degree (focused on studies of cell physiology) from Geneva University in Switzerland, he felt he belonged to Russia and therefore turned down a position at the Rome Botanical Institute in 1896. Unfortunately, his scientific degrees were not recognized in Russia and he had to repeat his studies there, at Kazan University, to receive his Master of Science degree (1901). He was appointed as an assistant lecturer of plant anatomy and physiology at Warsaw University in 1902, and the next year, on March 8, 1903, he presented a report, "On the New Category of Adsorption Phenomena and Their Application to Biochemical Analysis," at the meeting of the Botanical Section of the Warsaw Society of Natural Sciences. The invention of chromatography is now associated with this date. Tswett repeatedly reported on his technique on several botanical and chemical meetings, including international meetings, and published important papers in German. Surprisingly, however, he stumbled against an active opposition both in the West (Richard Willstätter) and in Russia (K.A. Timiryazev). Willstätter arranged to have Tswett's Doctoral dissertation, "Chromophylls in the Plant and Animal World," (1910) translated into German, yet continued to ignore and even criticize Tswett's dynamic adsorption technique. Willstätter was given the Nobel Prize in chemistry in 1915 and completely monopolized the field of plant pigment research in the West during that period.
World War I brought Tswett additional problems. Because of the German occupation of Poland in 1915 and of the Baltic "provinces" of Russia in 1918, Tswett lost all his personal and research belongings at Warsaw and Yur'ev (Tartu) Universities. He started enthusiastically working at Voronezh University, which was founded under one of the first Bolshevik decrees, but died from malnutrition and heart disease on June 26, 1919, soon after he turned 47. One cannot say that the scientific community was not yet ripe for accepting Tswett's ideas, but so many factors, both subjective and objective, prevented the successful start of chromatography until the 1930s and 1940s. Indeed, similar factors always strongly influenced the evolution of science.
The positive or negative role of personal factors can be traced even in the golden period of the development of chromatography, the second part of 20th century. An example is the failure of gas chromatography (GC) to contribute substantially to the thermodynamics of phase distribution, in spite of the fact that GC presents one of the most precise measurement techniques. My involvement in this subject goes back to the 1990s when, as head of the Scientific Council on Chromatography, I was asked by Russian colleagues to prepare a compendium of terms and concepts in chromatography. As usual, I started formulating definitions from my own understanding, but R.V. Golovnya pointed out that my formulation of such a fundamental term as "corrected retention volume" in GC contradicted the generally accepted formula. Looking it up in textbooks, I discovered that in 1952, Martin and James presented a correct equation for calculating this value, but did not explain the physical sense of the key component of this calculation, coefficient j23 . The name of this coefficient, "the compressibility correction factor," did not reveal its meaning, either. With the thermodynamic sense of the corrected retention volume remaining obscure, Littlewood suggested reporting these volumes at normal temperature, by multiplying the volumes by the temperature ratio 273/Tc, where Tc is column temperature. For more than 50 years this suggestion was recommended in all textbooks on GC and even in documents issued by the International Union of Pure and Applied Chemistry (IUPAC), and no one objected to the obvious nonsense of this additional correction: By going down from the high column temperature Tc to 273 K, the retention volume of any analyte must increase significantly, whereas multiplying by 273/Tc diminishes this value! I started a discussion in Chromatographia in 1996 on the thermodynamic meaning of the Martin and James gas compressibility correction factor. By deriving the j factor in a more logical way, I was able to show that it is none other than the ratio of the ambient pressure of the gas at the column exit to the pressure inside the column, averaged over the length of the column. From this, it becomes evident that Martin and James had suggested the formula for calculating the volume of the gas mobile phase under real chromatographic conditions — that is, under the column temperature and average pressure in the column — which makes the thermodynamic sense of the corrected retention volume crystal clear. Recalculating this value to standard temperature by using a totally inappropriate (for the thermodynamic retention parameter) coefficient, 273/Tc, was fundamentally wrong. This long-standing mistake practically killed the use of GC in physicochemical studies. Though IUPAC finally eliminated the error from its official documents (2), the erroneous traditional teaching will stay in GC textbooks for many years to come, unfortunately. On the contrary, GC flourished as an analytical technique as a result of the genius suggestion by Ervin Kovats to characterize an analyte not by the absolute value of the retention volume but rather by relative values, Kovats indices, where by dividing two retention volume values, both correction factors (j 23 and 273/Tc) cancel out.
Now that I am looking forward to my 75th birthday and looking back on my life, I realize that the net result of all the numerous obstacles and fortunate circumstances in my life and career placed me in the exciting swirl of the world's chromatographic community, although limited to participating only infrequently as a result of being on the other side of the Iron Curtain. In addition to the sheer luck of being born into a family of chemists, I drew another lucky ticket in 1957 when I was offered the chance to complete my high school education in China, Czechoslovakia, or the former German Democratic Republic. My choice was Germany, the country of chemistry, even though I understood that my personal life there would likely involve difficulties, because painful feelings from the recent terrible war were still vivid in every family in both the Soviet Union and Germany. As a member of the very first group of Soviet students, I arrived in Dresden. The city was in ruins, with the exception of its rich outskirts and the Technische Hochschule Dresden (the Dresden Institute of Technology). There, I diligently studied chemistry, and particularly enjoyed carrying out extensive experimental work. I happened to be the very first student of Gottfried Glöckner and under him, studied the living polymerization of styrene. (This brilliant person and scientist later developed the so-called precipitation chromatography of copolymers).
When I returned to Moscow in 1962, I was hired as a technician in the laboratory of Prof. Korshak of the Institute of Organo-Element Compounds of the Russian Academy of Sciences. Being rather conservative, as many Russians are, I am still working there today, 50 years later. Fortunately, I was not bound to any special research project and was allowed to pursue my own ideas.
Chiral Ligand Exchange Chromatography
Being unaware of manifold previous attempts to separate chromatographically enantiomeric (organic) molecules, I started enthusiastically synthesizing chiral ion exchangers on the base of slightly cross-linked styrene–divinylbenzene (DVB) copolymers. After chloromethylation of the latter, I enhanced the reactivity of the chloromethyl groups by exchanging chlorine atoms with iodine (by boiling the material with an acetonic solution of NaI) and then allowed natural chiral amino acids to be N-alkylated by the polymer. The chiral ion exchangers thus obtained were capable of partially resolving racemic amino acids into constituent enantiomers, but the enantioselectivity of the separation was far from impressive. A real success came in 1968 when I decided to switch from the simple ion-exchange mechanism to a new process that I dubbed chiral ligand exchange chromatography (CLEC). It was distinguished by introducing a copper(II) cation into the chromatographic system (3). The resin was first saturated with copper, which formed stable chelates with the carboxy and amino groups of the resin-bonded chiral selectors. The copper ion, which had a coordination number of 4, still exhibited the ability to additionally bind an amino acid molecule from the mobile phase. In the ternary mixed-ligand complex formed, the two amino acid ligands came into a dense interaction with each other and easily recognized the configuration of the partner. This resulted in unprecedented enantioselectivity in the ligand exchange process. Thus, the L-isomer of racemic proline was easily eluted with water from the column packed with the L-proline incorporating the polymer, whereas D-proline had to be replaced with ammonia in the mixed-ligand copper complex. Figure 1 presents this first example of a complete resolution of a racemate into two enantiomers by using liquid chromatography. Actually, the selectivity and loadability of the column correspond to those of the best modern preparative chiral stationary phases. The first complete resolution of a racemate on the analytical scale using capillary GC was reported by Gil-Av and colleagues just two years earlier, in 1966.
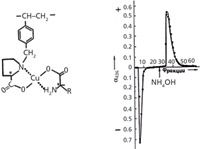
Figure 1: First case of a complete resolution of a racemate into enantiomers using liquid chromatography. Resolution of 0.5 g of D,L-proline on a column (475 mm à 9 mm) containing 11 g of copper(II)-saturated polystyrene-type resin with L-proline (2.3 mmol/g) as the chiral selector. Particle size: 30â50 µm; flow rate: 7.5 mL/h; detection: polarimetric. Abscissa, number of fractions. Adapted from reference 7.
CLEC proved to be a general approach to separating enantiomers that are capable of forming chelates with transition metal ions, that is, α- and β-amino acids, hydroxy acids, amino alcohols, diamines, but also some monodentate ligands with single functional groups (3).
An important theoretical question was raised concerning the enantiomeric purity of the chiral selector in the chromatographic system. I was the first to state that chromatography makes it possible to obtain a yield of 100% for both enantiomers in an enantiomerically pure state even when the chiral selector contains admixtures of the opposite enantiomer. However, Chemical Communications refused to publish this statement in my first international publication on CLEC (in 1971), because Eliel, in the popular book Stereochemistry of Carbon Compounds stated the opposite, namely, that the insufficient enantiomeric purity of the chiral selector unavoidably results in the lower enantiomeric purity (or yield) of the resolved enantiomers. In fact, both statements are correct, because Eliel was thinking of equivalent selector –selectand interactions whereas in chromatographic systems a large excess of the selector participates in the chiral resolution process. Several authors later proved the validity of my prediction in both chiral stationary and chiral mobile phase chromatographic systems.

Figure 2: Vadim Davankov (left) receiving the 1999 Chirality Medal from William Pirkle at the ISCD meeting in Chicago.
To elucidate the mechanism of chiral recognition in CLEC systems, the structure and properties of chiral model copper(II) aminoacidato complexes have been examined in detail. For the first time, thermodynamic enantioselectivity effects have been found in copper complexes with amino acids, diamines, and hydroxyketones, as well as in the mixed-ligand structures with these ligands. A unique example of a kinetic enantio-selectivity in the formation of copper(II)-diamine complexes was also discovered. These data strengthened the old supposition by Dalgliesh that chiral recognition requires a three-point solute–selector interaction, an idea that was repeatedly rejected by others. I recall an event that was unprecedented for scientific meetings: a hearing on that matter at the ISCD 1996 meeting in Edinburgh. Davankov took up the case against Prof. Irving Wainer, with all participants casting an official vote at the end. The chairman, Prof. William Pirkle, finally announced that the three-point model won.

Figure 3: Csaba Horvath and Vadim Davankov discussing CLEC in 1984.
The first person who noticed my publications on CLEC and invited me to give a seminar on the subject was Prof. Andrei Kiselev from Moscow University, an outstanding specialist in the field of silica-type adsorbing materials. My first chance to present the subject on an international level arose when I was invited by Harold Walton to attend the Gordon Research Conference on Ion Exchange (1977) in Wolfeboro, New Hampshire. It was there that I first met Klaus Unger. He advised me to try attending conferences on chromatography. Indeed, I received invitations from the 2nd Danube Symposium in Carlsbad in 1979 and then the 5th HPLC meeting in Avignon (1981). When announcing my lecture, the president of the symposium, Prof. Georges Guiochon, complained about the failure to extract Prof. Kiselev from Moscow, so I had to apologize for the problems in obtaining an exit visa from the USSR. I later had problems getting an entry visa to Italy in 1982 when I was invited to talk at the first conference on chiral separations before such distinguished participants as Gil-Av, Guiochon, Audebert, Jacques, Lahav, Pirkle, Blaschke, and Gasparrini. I managed to arrive only at the very last moment before my own talk. Totally confused and exhausted, I did not pay attention to the time and spoke more about peace and cooperation than chiral ligand exchange. That week, the meeting chairman, Prof. Misity, dared to invite to his home two people from opposite sides of the Iron Curtain, me and an American, Prof. Bill Pirkle. Bill was one of the first to enter the chiral chromatography business in the late 1970s, when he introduced chiral charge transfer chiral selectors bonded to silica. After a few glasses of wine, we could not avoid discussing politics. I said that next time, I would avoid visa problems by traveling abroad with a tank, just like Brezhnev. Bill and I became good friends for decades to follow.
The International Chromatography Community
In general, the chromatographic community was rather skeptical about the enormous enantioselectivity values (up to α = 15) reported in my papers on CLEC, so they did not pay much attention to my method. Therefore, I had practically no competition for almost a decade and was able to publish a lot of data in the 1970s and maintain my lead. The real boom in chiral chromatography started in the 1980s after the almost simultaneous introduction of silica bonded chiral ligand exchangers by three different groups, including ours, in 1979. Another significant improvement in the efficiency of CLEC resulted from the idea of converting conventional reversed-phase high performance liquid chromatography (HPLC) columns into highly efficient chiral phases by simple adsorption modification with N-decyl-L-hydroxyproline (4). This important paper resulted from my three-month stay with Klaus Unger in Mainz, Germany, where, for the first time in my life, I was able to use an HPLC instrument. The American company Regis Technologies asked me for permission to manufacture and distribute the new chiral columns under the name "Davankov columns" and then generously supported my participation in a series of HPLC and chirality meetings. In contrast, other companies have manufactured CLEC columns and thin-layer plates while disregarding my patents of 1968. Actually, my initial concept of CLEC was wrongly formulated in international patents as a chromatographic process that involves the participation of a transition metal ion in the sorbent–sorbate interaction. That claim was inappropriately formulated so that it addressed each individual user of the process, not the manufacturer of the chromatographic material.
It also should be noted that the CLEC principle was later used for the development of chiral mobile-phase-type separation techniques, chiral capillary electrophoresis, and even chiral preparative simulated moving bed systems. The growing international interest in CLEC gave me the opportunity to meet many other excellent scientists, including Dan Armstrong, Ernst Bayer, Hans Engelhardt, Leslie Ettre, Roland Frei, Georges Guiochon, Fred Helfferich, Gerhard Hesse, Csaba Horvath, Josef Huber, Henri Kagan, Rudolf Kaiser, Barry Karger, John Knox, Ervin Kovats, Wolfgang Lindner, G. Mannschreck, Milos Novotny, Yoshio Okamoto, Fred Regnier, Göran Schill, Volker Schurig, Eva Smolkova, Lloyd Snyder, Frank Svec, Nobuo Tanaka, Klaus Unger, and several others. I became good friends with many of them.
In 1985, I was given the opportunity to actively participate in the organization of the 5th Danube Symposium on Chromatography in the beautiful city of Yalta on the shore of the Black Sea in Crimea. An international meeting in the USSR was a rare event, and attracted the close attention of the Communist Party. This had both negative and positive consequences. On the one hand, I was instructed to reject contributions submitted by "adherents of Solidarnost" from Poland, since they would not be given entry visas anyway. I refused to follow this order. When a whole team from Poland showed up in Yalta, we had to solve the problem of their accommodations, because the hotels refused to accept them. On the other hand, we were given the magnificent Chekhov Theatre as the symposium venue and the newly built luxurious Oreanda Hotel to accommodate invited guests. One guest, Rudolf Kaiser, admitted that being in that hotel made him feel like a real kaiser. Among other important guests were Josef Huber, Bill Pirkle, Calvin Giddings, Ernst Bayer, Göran Schill, Ervin Kovats, and Fred Helfferich. We had the unique chance to visit the Massandra winery and hold a wine party in its cellars, which still preserve bottles of French wines that Napoleon left behind during his retreat from Moscow in the winter of 1812. We also were asked to plant trees in the Friendship Park of Yalta and attach plates on them with our names. I wonder how big that chromatography grove is now. At the closing session, we signed an open letter to the presidents of the Soviet Union and the United States, appeal ling to them to stop the arms race and nuclear tests. Those were Cold War times, when science and politics were closely intertwined. There were very few visitors to my country from the West and those visitors often felt uneasy. Cal Giddings composed a list of rules for foreign chromatographers visiting the Soviet Union. I still remember a few of them:
1. You do not exist other than in a group.
2. If there is a queue, join it.
2.1. If there are several queues, join the longest one.
3. "Minutuchku" means "wait a minute."
3.1. Be prepared to wait longer.
Some of Cal's advice might still be valuable today.
When many years later, in 2003, I organized an international symposium in Moscow called "100 Years of Chromatography," several participants approached me to say that they still had good memories of their first trip to the USSR. During that meeting in Moscow, we handed out copies of the marvelous book by Senchenkova, Michael Tswett: The Creator of Chromatography. For this, I am grateful to Leslie Ettre, who gave me invaluable help preparing the English edition of the book. The world's chromatographic community really is a family of friends. I am happy to be a member of it!
Polymeric Matrices for Column Packings
Russian scientists have never had modern instrumentation at their disposal (not to mention the deplorable state of fundamental research at the Academy of Sciences today). As a result, we are not in position to compete with our colleagues from the West in the application of modern science, including chromatography, to solve practical problems. To be useful to science, Russians have to be inventive and now and then come up with new ideas. I was lucky in this respect. When trying to improve the chromatographic efficiency of the chiral ligand-exchanging polymers, I suggested an original method for enhancing the permeability of the styrene–DVB-type matrix. This hydrophobic copolymer, even after having incorporated chiral amino acid ligands, did not swell properly in an aqueous mobile phase, so that intraparticle diffusion was slow. Swelling could be improved by introducing polar sulfonic acid groups, but I did not want to complicate the pure ligand exchange mechanism of chiral recognition. My suggestion was to let the initial styrene–DVB beads swell to a great extent in a good solvent like ethylene dichloride and then introduce many cross-bridges between the phenyl rings of the polystyrene chains. I wanted to convert the soft gel into a rigid open-network-type matrix, before providing the latter with chiral ligands. This idea went against all the rules, because additional cross-linking was known to reduce the swelling ability of any polymer. But it worked out and the efficiency of the final chiral resins was indeed much improved. None of the followers who tried to check my results on CLEC followed this recommendation, however, so they were able to confirm the high enantioselectivity of the CLEC systems, but not their high efficiency.
Soon, we recognized that hypercross-linked polystyrene with cross-linking densities greater than 50% was the first nanoporous polymeric material with an extremely useful complex of properties. This material had an apparently specific surface area that exceeded 1000 m2/g, was compatible with any type of mobile phase, and exhibited unprecedented adsorption capacities. After our patents expired, we were approached in the early 1990s by specialists from Purolite in the UK, and within a few months organized the large-scale manufacture of the new class of polymeric adsorbing materials, called "Hypersol Macronet." These materials found broad application in the purification of industrial aqueous and gaseous waste streams, in the recovery of valuable components, and in many other adsorption technologies.
The nanoporous structure of the hypercross-linked polystyrene has made it possible to develop a new process for the preparative (and even industrial-scale) separation of mineral ions — salts, acids and bases — by a size-exclusion mechanism. Electrolytes having larger ions (usually salts) are eluted ahead of electrolytes composed of smaller ions (usually acids or bases). Contrary to all known rules of chromatography, the selectivity of these separations increases with the concentration of the feed, and both separated components elute with concentrations higher than in the initial mixture (5). The process has great potential for hydrometallurgy and metal processing, such as titanium production.
In analytical chemistry, smaller particles of hypercross-linked polystyrene are the best material for preconcentration of trace compounds using solid phase extraction. They are available under several trade names, such as Purosep (Purolite), LiChrolute EN (Merck), and Isolute ENV (IST), and can be recognized by the advertised high surface area of about 1000 m2/g. It is not yet widely recognized that these materials are unique in that they permit extraction of not only hydrophobic compounds (which is also possible with reversed-phase silica), but also of highly polar compounds, like phenols from water or patulin from apple juice. Another new application area for these materials is preconcentration of aromatic and polar compounds from hexane solutions. The process greatly facilitates determination of polyaromatic hydrocarbons in oils and fats, or of polar furan derivatives in transformer oils. This property of hypercross-linked polystyrene is caused by its unique open-network structure that exposes aromatic systems able to enter p-p interactions with analytes containing lone electron pairs or p-bonds.
When prepared in the form of monosized microbeads, hypercross-linked polystyrene presents a novel HPLC column packing material that is compatible with any mobile phase and permits separations according to many different mechanisms, showing unique selectivities. These HPLC packings are still in the development stage, however.
As was the case with the CLEC, with hypercross-linked polymers we happened to be well ahead of partners from the West, so that at least 30 years passed before the topic attracted their close attention. More about these materials can be found in the monograph by Davankov and Tsyurupa, Hypercrosslinked Polymeric Networks and Adsorbing Materials (Elsevier, 2011). Once again, Leslie Ettre polished the English style of the book.
When looking back at my 50 years in stereochemistry, polymer science, and chromatography, I get excited about the wealth of new information that still remains to be discovered, even in popular areas of science that seem to be fully mature and thoroughly explored. But it should be noted that although some fundamental notions in science can and need to be revised, substantially new ideas need time to become established in the minds of scientists and in industry practice. Unfortunately, international scientific journals are rather conservative and it is hard to publish ideas that contradict commonly accepted notions. I could complain about the rejection of my manuscript presenting a new hypothesis about the origin of chirality of organic matter on the early Earth (6), but this would be a totally different story.
References
(1) E.M. Senchenkova, Michael Tswett: The Creator of Chromatography (English edition) V.A. Davankov and L.S. Ettre, Eds. (Scientific Council on Adsorption and Chromatography, Russian Academy of Sciences, Moscow, 2003).
(2) V.A. Davankov, Chromatographia 48, 71–73 (1998).
(3) V.A. Davankov, J.D. Navratil, and H.F. Walton, Ligand Exchange Chromatography (CRC Press, Boca Raton, Florida, 1988).
(4) V.A. Davankov, A.S. Bochkov, A.A. Kurganov, P. Roumeliotis, and K.K. Unger, Chromatographia 13, 677–685 (1980).
(5) V. Davankov, M. Tsyurupa, Z. Blinnikova, and L. Pavlova, J. Sep. Sci. 32, 64–73 (2009).
(6) V.A. Davankov, Russ. J. Physic. Chem. 83, 1247–1256 (2009), DOI 10.1134/S0036024409080019.
(7) V.A. Davankov and S.V. Rogozhin, Dokl. Akad. Nauk SSSR 193, 94–97 (1970).
Vadim Davankov, PhD, is the head of the Department for Stereochemistry of Sorption Processes at the Institute of Organo-Element Compounds of the Russian Academy of Sciences, Moscow 119991, Vavilov str. 28, INEOS, Russia, davank@ineos.ac.ru

Vadim Davankov, PhD

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









