Split-Vent Traps: GC’s Dirty Secret
LCGC Europe
In GC split injection systems as much as 99% or more of the injected sample never enters the column: It is released downstream of the inlet where it can encounter and possibly perturb precision gas control devices. The split-vent trap acts to prevent or at least moderate such effects. This month’s instalment addresses the operation and maintenance of split-vent traps.
In gas chromatography (GC) split injection systems, as much as 99% or more of the injected sample never enters the column: It is released downstream of the inlet where it can encounter and possibly perturb precision gas control devices. The split-vent trap acts to prevent or at least moderate such effects. This month’s instalment addresses the operation and maintenance of split-vent traps.
A plethora of gas chromatography (GC) injection techniques confront chromatographers in their daily work, and the related sample injection systems can be rather complex. Split, splitless, direct, programmedâtemperature, headspace, thermal desorption, and more all serve the purpose of transferring analyte species of interest into the separation column, and each does so in ways uniquely suited to the nature of the sample and its matrix. Beyond simply moving sample molecules from outside the column to inside, injection techniques share some additional requirements. First, they should transfer sample accurately and repeatably. Of course, these two terms don’t have significant meaning until they are cast in the light of analytical needs that define accuracy and repeatability. Another desirable trait is less-frequent maintenance and tolerance of a larger number of injections without a loss of performance. Although unavoidable in some extreme cases, no one wants to disassemble an inlet every few samples. The primary consideration for achieving the best possible injection performance is a close match between the sample, the injection technique, and the column. The analysis of traces of methylene chloride in polycarbonate beads, for example, is much better matched to headspace sampling than to direct or splitless injection. Headspace sampling leaves polymer residues behind in a disposable vial, while whole-sample vaporizing techniques such as direct, split, or splitless injection would rapidly contaminate the inlet and inlet liner, necessitating frequent maintenance. On the other hand, determination of polynuclear aromatic hydrocarbons (PAHs) is not well-suited to headspace sampling because of the high temperatures needed to volatilize the target compounds. Instead, the higher temperature ranges of vaporizing injection makes it a much better choice. But then vaporized sample components that migrate beyond the inlet may cause other problems.
Split Injection
Split injection helps match convenient and easy-to-dispense liquid sample sizes to the sample size and carrierâgas flow-rate requirements of open-tubular (capillary) columns. Liquid sample sizes of 0.5 µL or larger are best for handling by syringe; smaller injected sample volumes tend to compromise accuracy and repeatability because of evaporative and droplet-size effects. However, if the sample is relatively concentrated and it is all injected into the column, the total amount of solute may cause peak distortion because of overloading of the stationary phase. Split injection can reduce a too-large solute amount down to a volume the column can handle. Sample dilution is an alternative, but it may not be desirable as an extra sample preparation step or a source of possible contaminants. Split injection also reduces the amount of solvent in the column and avoids very large solvent peaks that might interfere with the separation of early-eluted solutes.
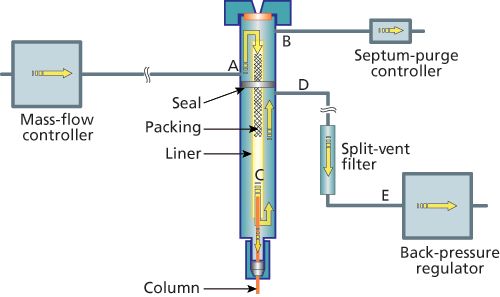
Figure 1 illustrates the basics of split injection with back-pressure regulated pneumatic control. A constant mass-flow controller provides carrier gas to the split inlet at flow rates from as little as 10 mL/min up to 500 mL/min or greater. This split flow enters the inlet at point A and then flows into the inlet liner. A small amount of carrier-gas flow is diverted upwards, across the septum, and out the septum purge vent B. The main carrier-gas flow passes through the inlet liner and past the tip of the column at the split point C. Some of the carrier gas flows into the column entrance, while the rest continues out of the inlet liner and exits the inlet at split exit D. The internal seal isolates the incoming and outgoing carrierâgas flows. The split ratio,
S
, is the ratio of the flow through the inlet liner to the flow rate of the column,
F
c
. Both flows must be expressed at the same temperature and pressure, normally at standard conditions of 0 °C or 20 °C and 101 kPa. The liner flow is equal to the total flow through the inlet,
F
s
, minus the split-vent flow,
F
v
:
S
= (
Fs
–
F
v
)/
F
c
[1] The split ratio would be 100:1, for example, with a column flow rate of 1.5 mL/min, a total split flow of 152.0 mL/min, and a split-vent flow of 2.0 mL/min.
The split ratio refers to the ratio of the flows in equation 1 under static conditions and not to the actual fractional amount of sample that enters the column. The injection process itself is far from static. Upon entering the inlet liner, solvent and sample undergo rapid heating and expansion, which causes a pressure and flow impulse to develop and propagate along the liner in both directions. Note that this is not the “pressure pulse” of pressureâpulsed split–splitless injection, a technique that purposely increases the inlet pressure setpoint before injection and then reduces it back to normal after injection is complete. This higher initial inlet pressure can help narrow the injection pulse and control some of the effects of vaporizing injection. Column flow momentarily increases as the pressure impulse encounters the column entrance, but because of the relatively high restriction of the column and low restriction of the inlet liner and other inlet internal passages, the split flow will not react proportionately and the split ratio will deviate away from its initial quiescent value. The amount of each solute that enters the column is a complex function not only of the solvent, inlet temperature, injection amount, and inlet flow rates, but also of the liner packing and configuration, solute volatility, and injection speed. For these reasons, the split ratio should be taken as an approximation, and actual solute transfer into the column should be determined by careful calibration, along with recalibration upon modification of any aspects of the injection.
Split-Vent Trap
After leaving the inlet at the split exit D, carrier gas passes through a split-vent trap, then through a back-pressure regulator, and finally is vented to the surrounding air. The split-vent trap serves two main functions. First, it acts as a buffer between the inlet and the backâpressure regulator that reduces the size of the injection pressure impulse that reaches the regulator. Second, it traps and holds lessâvolatile sample components that could otherwise contaminate the regulator and affect its operation permanently. An additional benefit is the trapping of toxic substances so they are not released into the laboratory atmosphere. An optional external split-vent trap, installed after the back-pressure regulator, at the external split-vent exit connection, can help ensure that toxics do not leave the instrument. Such external traps are useful if solvent exposure is a concern as well. The injection pressure pulse reaches the back-pressure regulator quickly, while sample splitting is still occurring inside the inlet. The regulator will sense the pulse and open itself up a bit to relieve the increased pressure. As the pressure pulse subsides, the regulator will head back to its previous static position. These fluctuations add to the carrier flow perturbations caused by injection and can increase uncertainty in the injected solute amounts as well as change the retention times of early-eluted solutes. By including the extra volume of the split-vent trap in the flow path, the extent of the pressure impulse is reduced, and it is spread out over a longer period. Fluctuations in the regulator are mediated and the inlet pneumatic system is made more stable. Using larger diameter tubing between the split exit D and the split-vent trap also helps mediate the pressure impulse. The initial injection pressure impulse propagates rapidly from the split-vent exit, at the speed of sound through the carrier gas and tubing. Shortly afterwards, moving at the split-vent flow rate, sample components and solvent start to move out of the inlet. In the absence of the splitâvent trap, these would reach the backâpressure regulator within about 1 s after the start of injection and would further perturb its operation. Solvent vapour has a higher viscosity than carrier gas, and its presence at the back-pressure regulator would cause the regulator to open even further in compensation. The split-vent trap entrains solvent and sample components that are vented from the inlet, keeping them from entering the regulator immediately. With a split ratio of 100:1, this amounts to 99% of the total sample amount. Solvent will eventually break through the trap and be released slowly into the backâpressure regulator; over time most solvents will be purged from the trap. Less-volatile solutes will tend to accumulate, so that the backâpressure regulator is protected from the solutes condensing inside.
Symptoms and Maintenance
Many chromatographers tend to neglect their split-vent traps, which are hidden underneath instrument panels and are easy to forget while troubleshooting or maintaining inlet systems. If normal maintenance procedures of replacing the inlet liner, internal seals, and septum do not remedy problems such as peak tailing, poor accuracy, and poor repeatability, or inability to achieve high split ratios, then the split-vent trap or connecting tubing may be at fault. Before replacing the split-vent trap, carefully check the split-vent and septum purge flow rates, preferably with an accurate digital flowmeter, and make sure there are no leaks around the inlet seals and septum. Leak checking is best done with an electronic leak sniffer and hydrogen or helium carrier gas. Using a surfactant solution on a cooled-off inlet will also find major leaks, but may then create a major headache because the solution can be drawn into the inlet by capillary action back through a leak. A blocked or restricted septum purge is a sure sign of a repeatedly overloaded inlet and the accompanying back-flash of sample up into the septum area. This problem is always accompanied by corresponding downstream contamination in the direction of the split-vent trap. While most common with splitless injection, low split ratios can yield the same effects. It may be necessary to replace the inlet nut assembly in this case, and if so be sure to replace the split exit tubing and split-vent trap as well. In extreme cases it may be necessary to remove the inlet entirely and solvent clean it. If the septum purge flow is normal, meaning about 2–5 mL/min, then the problem may be a routine buildâup of contamination in the split-vent trap. The trap may last for up to a year if injections are primarily clean volatile samples with little to no high-boiling residues. Samples that contain high levels of nonvolatiles, on the other hand, could necessitate trap replacement as often as monthly. With a replacement trap or trap cartridge on hand, proceed to remove the existing split-vent trap by following the instrument manufacturer’s procedures. With the trap removed, inspect the incoming tubing, the connections, and the trap itself for any visible residue. If found, such residue indicates the connecting tubing back to the inlet also needs replacement. This may be best performed with a service visit by an authorized technician. Consider modifying sample preparation in such cases so that as little high-boiling material as possible is injected. Be sure to leak-check the new connections and verify the correct split-vent flow rate, then try again to achieve good chromatographic accuracy and repeatability. Other injection systems also have vent lines that conduct sample components to the outside. Many headspace samplers, for example, vent a large portion of the sample. Thermal desorption systems usually have at least one split point as well. I remember one site visit where several headspace samplers were in use. Every one of them had a blocked vent exit tube, as evidenced by a complete lack of flow when the vent valve was activated manually. The sample was not particularly dirty, but the material under analysis, which needed an elevated thermostating temperature for good headspace results, had been condensing in the final short, unheated, section of vent line tubing for an extended period.
Conclusion
Split injection vent traps are a necessary adjunct to high-performance gas chromatography with openâtubular columns. These traps protect carrierâgas regulation components in the short term from performanceâdegrading perturbations because of injection, and in the long term from accumulation of sample residues that can also affect performance. Split-vent traps also help prevent nonvolatile toxics from entering the laboratory atmosphere. Often, however, split-vent traps are not serviced and maintained as regularly as they should be. Residues can build up rapidly when analyzing many samples with heavy components, and contamination can sometimes extend back into split-vent connecting tubing. Careful attention to all of the inlet components, including splitâvent and septum-purge elements, can significantly improve results and reliability. “GC Connections” editor
John V. Hinshaw
is a Senior Scientist at Serveron Corporation in Beaverton, Oregon, USA, and a member of
LCGC Europe
’s editorial advisory board. Direct correspondence about this column should be addressed to “GC Connections”,
LCGC Europe
, Hinderton Point, Lloyd Drive, Ellesmere Port, Cheshire, CH65 9HQ, UK, or email the editor-inâchief, Alasdair Matheson, at amatheson@advanstar.com

This information is supplementary to the article “Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing”, which was published in the May 2025 issue of Current Trends in Mass Spectrometry.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).
Evaluating Natural Preservatives for Meat Products with Gas and Liquid Chromatography
April 1st 2025A study in Food Science & Nutrition evaluated the antioxidant and preservative effects of Epilobium angustifolium extract on beef burgers, finding that the extract influenced physicochemical properties, color stability, and lipid oxidation, with higher concentrations showing a prooxidant effect.







