Let’s Do It Right the First Time: The Importance of Solvent Safety Considerations
LCGC Europe
Given some recent, significant safety incidents, this month we step back and take a quick refresher on safety concerns to be aware of during our sample preparation activities.
If sample preparation is the most time and labour intense step in the analytical process, and uses the largest amount of solvents, it stands to reason that sample preparation may present the most significant safety risks in the analytical laboratory. While most laboratory workers receive significant safety training, we may become numb to the prospect of accidents or get into the mindset that accidents only happen to other people. Given some recent, significant safety incidents, this month we step back and take a quick refresher on safety concerns to be aware of during our sample preparation activities.
When I was first approached about taking on the “Sample Prep Perspectives” column, I sought the advice of “LC Troubleshooting” columnist John Dolan. I asked, “How do you come up with fresh ideas each month?” John replied that he often gets e-mail queries from readers that provide the basis for some of his instalments. That’s finally happened to me. Earlier this summer, someone from a large chemical company wrote me about guidance concerning solvent safety and their new safety programme. Safety issues are simultaneously overlooked and are seeing a resurgence of interest. Wellâpublicized laboratory accidents are leading university and research institutes to place even greater scrutiny on safety. Yet, at the same time, a trusted colleague working in a top-tier analytical department confided to me the complete lack of safety concerns at their new workplace. Since solvent use is a major contributor to safety concerns with extractions and sample preparation, this month we take a look at these considerations. Note that we will not be looking at alternative solvents and replacements - that’s a topic for another column. Here we will focus on safety aspects with traditional molecular solvents. As one would expect, there is a wealth of information on solvent safety considerations in the literature and on the internet, but finding this information in a concise format is somewhat more difficult. One important resource, though not oriented toward analytical use, is the European Solvents Industry Group (www.esig.org), including the presentation of a series of “Best Practice Guides”.
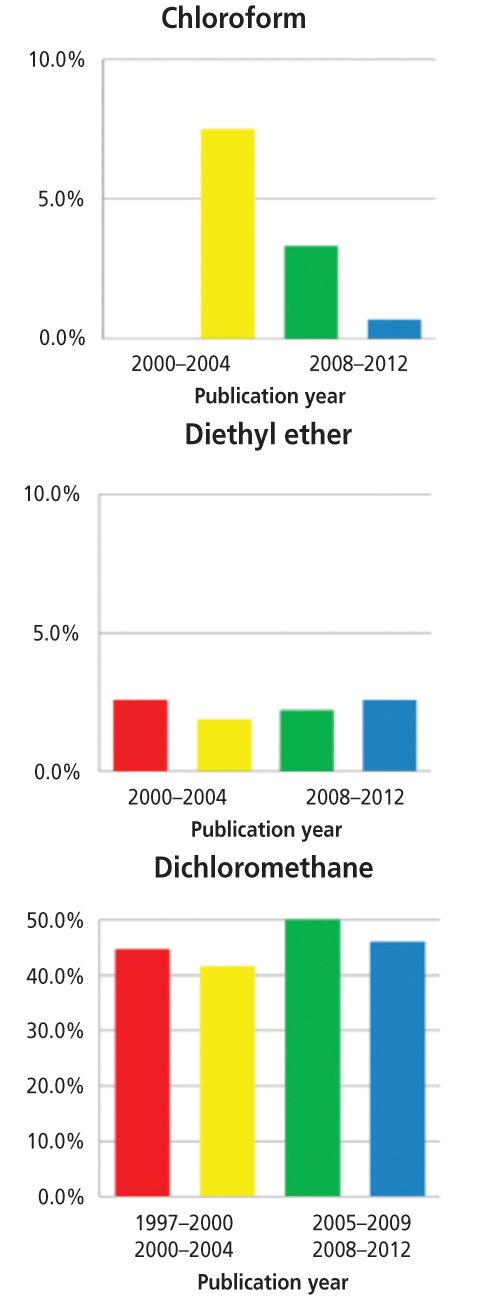
It is unquestioned that extractions generally use the largest amounts of solvents in analytical methods. Anastas (1) has pointed out that waste solvents from Environmental Protection Agency (EPA) laboratories tend to be ignitable, corrosive, or toxic. Furthermore, 86% of EPA laboratory wastes are Resource Conservation and Recovery Act (RCRA) F-listed, or multicode, solvents, including acetone, ethyl acetate, methyl isobutylketone, methanol, toluene, and dichloromethane. Some concern over the nature of organic solvents has been noted. For example, a study of papers in the journal
Organic Process Research & Development
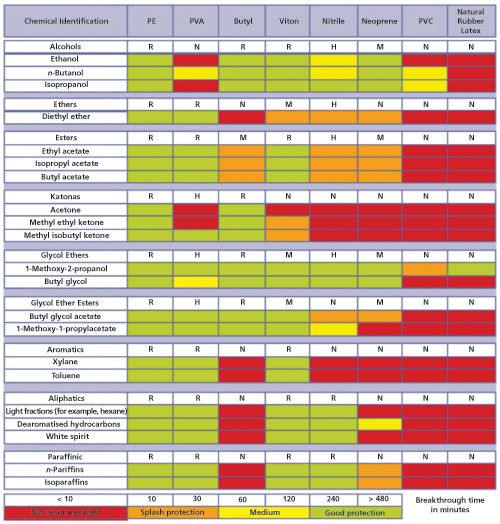
(2) found that between 1997 and 2012, the use of chloroform and n-hexane significantly declined, but the use of ether and dichloromethane remained somewhat constant (see Figure 1). In reporting these results, I only considered solvents of interest for analytical extractions and recognize that solvent considerations for synthetic organic processes are different from analytical extractions. The analytical community has broadly explored the use of alternative solvents like supercritical fluids and ionic liquids, but given the use of regulatory and other standard methods, the widespread use of molecular solvents still predominates. The solvents often used in analytical extractions are typically flammable and possess a diversity of toxicological concerns, including skin irritation or defatting, nervous system depression, reproductive effects, and other acute or chronic effects. Regarding the flammability of organic solvents, flash points can be used to predict the risk associated with solvents (flash points greater than 27–57 °C are suggested for laboratory solvents), though data is missing with mixed solvents. Liaw and colleagues (3) determined that for binary mixtures the flash point is intermediate between that of the component solvents, though in most cases, phase equilibrium has not been achieved. The liquids themselves do not burn, but rather the solvent vapours are flammable. Meanwhile, Klein, King, and Kosior (4) investigated the accumulation and clearance of solvent vapours in the laboratory. They found that chemical vapour pressure and density, room temperature, and direction and velocity of room air currents influenced contaminant levels. While no definitive statement could be made, room air change per hour rates of 6–8 are generally recommended. Keeping in mind that safety considerations with extraction solvents depend on both the property and nature of the solvents and the exposure to the solvents, important guidelines can be compiled as follows: • â¨Laboratory guidelines should be documented in standard operating procedures (SOP) or similar means. • â¨Consult the supplier’s safety data sheet for specific concerns on a given solvent. Ethers and some nonaromatic unsaturated cyclic hydrocarbons may form peroxides, which can be explosive. Do not allow these solvents to be evaporated to dryness. (Note: Petroleum ether is a distillate cut; it is not an ether.) • â¨Restrict laboratory access to only trained staff with a reason to be there. All laboratory staff should be trained on appropriate safety considerations regarding working with solvents. • â¨Handle solvents inside fume hoods whenever applicable. Maintain appropriate air flow within the fume hoods. ⨕ Clean up solvent spills immediately. Approved spill kits should be readily available in the laboratory. • â¨Replace lids on solvent containers after use. ⨕ Store solvents in approved containers, using secondary containment as needed, and dispose of empty containers according to local policy. Generally, solvents should be stored below eye level. • â¨Keep personal protective equipment clean and replace at recommended intervals and as appropriate. Glove material should be appropriate for solvent used (see Figure 2). • â¨Ignition sources, including open flames and static discharge sources, should be well isolated from solvent use areas. • â¨Do not use solvents to wash hands or skin. ⨕ Solvent bottles should be dated when they are opened. • â¨Waste solvent disposal should follow your organizations guidelines and applicable regulations.
Figure 2: Some examples of solvents with the most chemical resistant glove materials (excluding single use) for protection. (Adapted from www.esig.org with permission).
Additional Comments
Please note a few more remarks about the solvent compatibility of gloves: ⨕ The resistance of the materials will be dependent on the thickness of the gloves, temperature, and many other environmental factors. • â¨The recommendations given are based on laboratory testing with pure chemicals. Check with the glove manufacturer for specific applications. • â¨Glove manufacturers have databases with test results of their gloves against many chemicals. This information is available from your supplier; make sure that you check your specific product and application. Gaber and colleagues (5) created a free software program, HPLC-EAT, that evaluates the environment, health, and safety of liquid chromatography mobile phases. The program is available for download in the supplementary material at: http://pubs.rsc.org/en/Content/ArticleLanding/2011/GC/c0gc00667j#!divAbstract. Presumably, this information could also be applicable to extraction solvents. In addition to the advice provided above, standard safety precautions not exclusive to working with solvents should be followed. These include the use of safety glasses (goggles), regular inspection of eyeâwash stations, and so on. When developing laboratory safety guides for your laboratory, consult similar guides that may be publically available. Those developed by the University of Wisconsin and Yale University have been recommended to me. Specific extraction techniques may have additional safety considerations, such as those involving the use of glassware, heat, high pressure, or sharp needles, and should also be addressed.
Conclusion
Solvents represent probably the largest source of safety concerns in sample preparation procedures. While laboratory supervisors and workers receive training in the proper use of solvents, the results of this training are not always put into practice. Guidelines for developing safe practices were presented here that can be used to assist organizations in developing, or reinforcing, a culture of laboratory safety.
References
(1) P.T. Anastas, Crit. Rev. Anal. Chem.29, 167–175 (1999).
(2) C.P. Ashcroft, P. Dunn, J. Hayler, and A.S. Wells, Org. Proc. Res. Dev. 19, 740–747 (2015).
(3) H.-J. Liaw, V. Gerbaud, C.-C. Chen, and C.-M. Shu, J. Hazard. Materials177, 1093–1101 (2010).
(4) R.C. Klein, C. King, and A. Kosior, J. Chem. Health Safety16, 36–42 (2009).
(5) Y. Gaber, U. Tonvall, M.A. Kumar, M.A. Amin, and R. Hatti-Kaul, Green Chem.8, 2021–2025 (2011).
“Sample Prep Perspectives” editor
Douglas E. Raynie
is an Associate Research Professor at South Dakota State University, USA. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee. Direct correspondence about this column should be addressed to “Sample Prep Perspectives”,
LCGC Europe
, Hinderton Point, Lloyd Drive, Ellesmere Port, CH65 9HQ, UK, or email the editorâin-chief, Alasdair Matheson, at amatheson@advanstar.com

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).








