A Split Decision
LCGC Europe
This month's installment of "GC Connections" discusses factors that can help determine which type of liquid injection technique is appropriate for specific samples, including having to use existing inlet systems that are on-hand in the laboratory with samples for which they might not be ideally suited.
Gas chromatography (GC) inlets transform a sample from its physical state outside the chromatograph into a state suitable for separation inside the column. This transformation consists of two principal steps: transfer from the outside into the inlet and transfer from the inlet into the column. On the way to the column, the sample undergoes volume, concentration, temperature, and pressure changes that convert it into a condition that is more or less compatible with the separation that follows. Chromatographers measure an injection's success by the degree to which it preserves the relative sample composition while not interfering with separation, and by how well the injection process repeats from run to run.

John V. Hinshaw
In GC, samples can be liquids, solids, or gases. The type of sample and its concentration, taken along with the column and detector, determine which types of injection will give the best results. There often is more than one choice of appropriate injection technique, of which only one might be available in a particular laboratory or instrument. Sometimes none of the suitable inlet systems is available, in which case the analyst might have to choose between compromising the injection or upgrading their chromatographic equipment. Where they have the methodological latitude, they also can solve this dilemma by modifying preparation procedures to make the sample compatible with available inlet systems, or by choosing a different, more compatible column. For example, analysis of a trace-level sample might best be performed with splitless or on-column injection onto a 25-m, 0.25-mm i.d. high-resolution column. Without an on-column or split–splitless inlet on-hand, the chromatographer could choose to sacrifice speed of analysis by using a longer 60-m, 0.53-mm i.d. megabore column with the same phase ratio and direct injection. Such a column could produce about the same resolution as the narrow-bore column in about twice the time, and it is compatible with the larger injection volumes produced by direct injection.
Liquid sample injection techniques for open-tubular (capillary) column GC can be classified by, among several variables, the fraction of the vaporized sample that enters the column. For trace-level analyses, it is desirable for all or at least a substantial portion of the sample to enter the column to maximize method sensitivity. But at higher concentrations the amounts of analytes that traverse the column can become large enough to affect separation quality by causing peak overloading and peak shape distortion, so in these cases only a fraction of the sample is passed into the column by using split injection. To complicate the situation, passing liquid sample volumes greater than about 1 μL directly onto the column can cause a number of solvent-induced side effects that detract from the column's resolving power: controlling these effects can be critical. Often, it is very clear which type of injection technique will be the best choice for a specific sample and analysis, but there are many samples that could be handled by multiple injection types. Deciding which to choose can be difficult, and understanding the side effects that can occur is vital for troubleshooting inlet problems.
Range of Analysis
The total dynamic range of analysis that a given GC system can handle while maintaining peak resolution and quantitation is constrained by inlet, column, and detector considerations. The range of sample concentrations that ionization detectors for GC can handle is quite large — as much as nearly seven orders of magnitude, from around 2 × 10-12 g/s to 1×10-5 g/s for example, with flame ionization detection (FID). Electron-capture detection (ECD) and mass spectrometric (MS) detection have even lower detection limits. Before the detector, however, the column itself also has minimum and maximum sample concentration levels that it can tolerate. At the high end, too much sample can distort peaks due to overloading effects, even though the detector has no difficulty transponding the large peaks. Table I lists measured upper limits for solute amounts on several different columns. The solute and the column temperature also strongly influence these limits.

Table I: Measured sample capacities for four 25-m long open-tubular columns with methylsilicone stationary phase. Solute: n-undecane. Sample capacity is defined as the amount corresponding to a 20% reduction in theoretical plate number (1)
At the low end, the column might not pass trace levels of some analytes through to the detector due to adsorption or catalytic decomposition, especially of polar or thermally labile components, though the detector itself is sensitive enough to respond. There also are limits on the minimum and maximum sample volumes as well as on the minimum concentrations that various inlet systems can handle. Too much liquid sample volume, for example, can overload a splitless inlet and cause sample vapor to flash back into the carrier gas supply lines, while too little volume can be difficult to inject repeatedly within required reproducibility levels. Thus, the overall dynamic range of the inlet–column–detector system is limited at both the high and low ends by the characteristics of each link in the chain that the sample encounters as it passes through a gas chromatograph.
Split Injection
One of the most common inlet-related problem sources for gas chromatographers is selecting and using a suitable inlet for a specific sample type. Table II matches common inlet systems and injection techniques with various sample types and detectors.
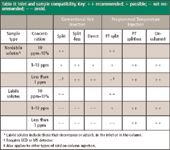
Table II: Inlet and sample compatibility. Key: + + recommended; + possible; â not recommended; â â avoid.
In split injection, only a fraction of the injected sample enters the column; the rest is discarded. Conventional vaporizing split injection rapidly flashes liquid sample to the vapor state inside a heated injector that is held at a constant temperature, ostensibly greater than the solvent boiling point. A split flow arrangement passes a small fraction of the vaporized sample into the column while the remainder is vented to the outside. The split ratio refers to the fraction that enters the column out of the total carrier gas flowing through the split inlet system: if the split ratio is 100:1, then 1 % enters the column and the rest is discarded. Split injection is useful when sample concentrations are too high, such that injecting even a small volume would overload the column. One microliter of a 1000 ppm w/v (parts-per-million on a weight per volume basis; 0.1% w/v, or 1 mg/mL) solution, for example, contains 1000 ng solute, which is far too much to inject onto any but the thick-film 530-μm i..d column listed in Table I. Split injection with a 50:1 split ratio brings the solute amount down to 20 ng, which is a reasonable amount for the 0.25-mm i.d. thin-film column.
Split ratios can range as high as about 1000:1 for GC columns with relatively low carrier gas flow rates. For example, a 25 m × 0.10 mm microbore column running with an average carrier gas linear velocity of 30 cm/s of helium carrier gas, at around 80 psig inlet pressure, would have a mass flow rate close to 0.5 sccm. In this case a 1000:1 split ratio would call for a total split flow rate of 500 sccm, which is about the highest practical split flow rate that can be attained with commercially available GC inlet systems. Such a column, with an upper solute mass limit of around 1 ng, could tolerate a 1-μL injection at a 1000:1 split ratio for a maximum sample concentration of 1 part-per-thousand under these inlet conditions.
A different situation arises at higher capillary column flows: the required total split flow can be high enough so that the inlet pneumatics can loose control of the inlet pressure. For example, a 10 m × 0.53 mm thick-film column running around 40 cm/s will consume about 4–6 sccm with an inlet pressure of about 1–1.5 psig. Suppose a high-concentration 10% sample is to be injected. A split ratio of 100:1 with 0.5 μL injected will place about 0.5 μg onto the column, which is a high but reasonable amount for the column. The resulting total split flow would be 400–600 sccm, close to the maximum flow. The problem is that this high of a total flow running through the split pneumatic system will generate a "natural" back pressure that exceeds the desired column inlet pressure, and this in turn will cause the gas chromatograph to enter a not-ready state and possibly trigger an inlet leak fault condition. In such cases, even though the column might tolerate the high injected solute amounts, it is better to dilute the sample, thereby reducing the required split ratio and allowing the pneumatics to run at a lower split flow rate. In this case, the split flow should be less than about 100 sccm so the maximum useful split ratio is more like 25:1.
Injecting less than 0.5 μL of liquid sample, to limit solute amounts that get onto the column, is not recommended for a couple of reasons. First, this is approximately equal to the needle volume of a conventional 10-μL microsyringe, and in a vaporizing inlet, it is not practical to inject less. Second, even with a plunger-in-needle design syringe that has next to zero residual needle volume, the reproducibility of smaller injections can suffer from incomplete transfer of sample from the syringe needle into the inlet. In addition, when the solutes span an appreciable range of volatility, they might be fractionated, or distilled, from the syringe needle so that they do not reach the column in proportions that represent the original sample. A high-speed autosampler and larger injection volumes help correct this effect.
As sample concentrations decrease, split ratios can be decreased proportionately, down to a minimum practical limit of about 10:1, depending upon the column. It is good practice to select a split ratio that lands the solute amounts entering the column at the higher end of the column's working range. One microliter of the one part-per-thousand, for example, could be split at a ratio of 50:1 onto a column that could handle 20 ng of solute. It is desirable in such cases, if there is overhead for more solute, to increase the injection volume to 1 μL, as this yields better repeatability.
Solute amounts delivered to the column by split injection become quite small as concentrations approach 100 ppb (parts-per-billion; one part in 109); in such cases it is tempting to decrease the split ratio to less than 10:1 and to increase the sample volume beyond a couple of microliters. Split inlet systems have some limits at the low end, however, which restrict operation with trace-level samples while still performing split injection. First, most split injectors can control the split flow rate down to about 20 sccm. Below that level, there is simply not enough gas flowing to split and purge the sample effectively; also, if the velocity at which the sample traverses the inlet drops to a fraction of the inlet velocity of the column, early-eluted peaks can become overly broadened. In lieu of a lower split ratio, it is tempting to increase the injected volume. But, due to the slow split flow rate, the problem of sample vapor backflowing into the pneumatics becomes more significant. Overall, it is better to switch to splitless or on-column injection unless proscribed by methodology or equipment availability.
Another difficulty with trace-level injection can be the adsorption or decomposition of sensitive solutes. In conventional split injection, all solutes approach the high inlet temperature rapidly as the sample is flash-vaporized. This thermal stress can break down some components, or can activate inner surfaces exposed to the sample and cause adsorption. With the alternative programmed-temperature vaporizing (PTV) split injection, the inlet temperature increases gradually over 0.5–1 min, which allows solutes to vaporize and exit the inlet system without encountering excessively hot temperatures and also can help control adsorption. This does not help much with higher-boiling solutes that have to be heated up anyway, but it might make a significant difference in many cases. PTV injection also largely avoids problems with sample vapor flooding the inlet system and backflowing into the inlet's pneumatic system.
Splitless and On-Column Injection
For trace-level samples, splitless and on-column injections are the techniques of choice. Splitless injection is, of course, a misnomer: it might better be named "delayed-split" injection. It is performed on conventional split inlet systems with the addition of the capability to stop the split vent flow momentarily. In operation, the injected sample is vaporized—either rapidly in the case of conventional vaporizing splitless injection, or more gradually in the case of PTV splitless injection. During the vaporization period and for some time afterwards, the split vent flow is stopped or bypassed around the inlet. This forces the vaporized sample to flow only into the column. After a delay of about 1 min or more, the split vent flow is re-established and any remaining sample vapors are flushed out. The idea is to arrange the timing and the inner volume of the inlet so that the majority — preferably 90% or more — of the sample enters the column before the split vent flow is restored. The major advantage of PTV splitless injection is the same as for PTV split injection: the sample is exposed to the minimum temperatures required to vaporize it as opposed to the temperatures required to vaporize all of it at once. However, in some portion of PTV splitless, the sample is exposed to elevated temperatures for longer periods of time due to the delay in the split vent flow.
With 90% or more of the sample entering the column, splitless injection is ideally suited for trace level analysis, and concomitantly not suited at all for higher concentration samples. The upper limit of sample concentrations for splitless injection is the same as the limits on the column itself. 1 μL of a 100-ppm sample, for example, contains 100 ng of solute, which is right at the upper limit for conventional thin-film columns. Thick-film columns will tolerate perhaps 10-fold higher concentrations, but microbore columns present their own challenges in splitless injection. Due to their very low flow rates, the time to transfer the majority of sample vapor from inside a splitless inlet into a microbore column can be many times longer than a practical splitless injection time period. A low-volume inlet liner helps speed the transfer, but at the same time suffers from a reduced maximum sample volume. In any case, temporarily increasing the inlet pressure during the splitless sampling period before turning on the split vent flow — the so-called "pressure-pulse" injection technique — helps to transfer sample into the column more effectively.
On-column injection delivers about the same amounts of sample to the column as does properly configured splitless injection. The difference is that in on-column injection, the only gas flow is through the inlet and into the column — there is no split flow to consider. Generally, the syringe needle enters the column directly, although a precolumn (retention gap) often is used to provide the equivalent functionality of the splitless inlet liner in terms of a removable location from which liquid sample moves and evaporates into the separation column itself. On-column injection performed at high temperatures is usually termed "direct" injection, to distinguish the two. So-called cold on-column injection is the more common variety, in which the temperature of the area in which the syringe deposits the sample roughly follows the GC oven temperature. In PTV on-column injection, the sample landing area is temperature-controlled separately from the GC oven, which gives some additional flexibility for specialized injections where necessary.
The principal advantage of on-column injection is that the sample encounters only the syringe and column or precolumn. The degree of adsorption and decomposition is reduced greatly compared to splitless injection as well as to PTV splitless in many cases. Mechanical issues with manipulating the syringe needle into the column itself make the use of a precolumn desirable, but even then full automation is more challenging than with conventional split or splitless injectors.
At the low end, as sample concentrations drop below 1 ppb, solute amounts entering the column can be small enough that adsorptive effects on the column wall itself, or on contaminants left from previous injections, come into play. This is especially true with thinner stationary phase films. While column manufacturers have made great strides in increasing the inertness of column tubing, a good evaluation of splitless quantitation across a method's full range of target concentrations will reveal any initial problems. Regular monitoring over the lifetime of a column helps uncover any developing problems.
One remedy for difficulties with very small solute amounts is to increase the injection volume and raise solute levels in the column. However, injection volumes of greater than 2–5 μL, or even less if the inlet liner internal diameter is small, might allow significant amounts of solvent and volatile solute to enter the pneumatics as well. Also, landing large solvent volumes in the separation column can cause serious problems with peak distortion. Specialized PTV techniques with solvent venting arrangements have been applied to this situation, which make it possible to inject greater than 10 μL in some cases, but these methods are beyond the scope of this article.
Conclusion
The panoply of injection techniques for modern capillary GC can be confusing when trying to choose an injection technique, troubleshoot a problem, or find a suitable substitute when the primary choice is not available. Understanding how the various techniques work, and how they are interrelated, can help chromatographers make more informed choices.
John V. Hinshaw "GC Connections" editor John V. Hinshaw is senior staff engineer at Serveron Corp., Hillsboro, Oregon, and a member of LCGC's editorial advisory board. Direct correspondence about this column to "GC Connections," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com
For an ongoing discussion of GC issues with John Hinshaw and other chromatographers, visit the Chromatography Forum discussion group at http://www.chromforum.com.
References
(1) W. Seferovic, J.V. Hinshaw, and L.S. Ettre, J. Chromatogr. Sci. 24, 374–382.
Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.














