Solid-Phase Microextraction
LCGC North America
SPME decouples sampling from matrix effects that would distort the apparent sample composition or disturb the chromatographic separation. It is also easy to use and reduces solvent consumption.
Gas chromatography (GC) resolution and sensitivity are often limited by matrix effects in "real world" samples that originate outside of a laboratory. GC separation and detection may encounter interference from nonvolatile constituents, side-effects of large sample volumes, and the chemical activity of matrix compounds and derivatization residues. A number of sample preparation techniques increase analyte concentrations and detector response while reducing matrix effects, including classical liquid–liquid extraction, chemical derivatization, and sample preconcentration, as well as headspace, thermal desorption, and large-volume sample injection.
Solid-phase microextraction (SPME) is a relatively new sample extraction technique — first described in the 1990s by Pawliszyn (1,2) — that brings some unique capabilities to bear on the chromatographic analysis of dilute solutions in difficult matrices, both liquid and gaseous. Essentially, SPME consists of two discrete steps: solute absorption from the sample matrix into a thick layer of silicone or related adsorptive material, followed by transfer of the absorbed analytes into a chromatography inlet system by thermal or liquid desorption.
SPME has been applied to both GC and liquid chromatography (LC) separations. It eliminates the need for large-volume sample transfer into a GC column by concentrating analytes into the fiber coating while leaving the bulk of the solvent and nonvolatile residues behind. SPME uses orders of magnitude less solvent and has significant potential to greatly reduce or eliminate solvent consumption and the concomitant issues of used solvent disposal as part of sample preparation.
Chromatographers should not confuse SPME with solid-phase extraction (SPE), a related predecessor with similar applications. The principal difference is that SPE is carried out with a relatively large sorptive wafer the size of a small filter paper and requires post-sorption liquid-phase extraction of analytes; SPME is accomplished with a small fiber or tube coated with sorptive material. SPME is applied to both gas-phase and liquid-phase extraction, whereas SPE is limited to extraction from liquid-phase samples. This GC Connections installment primarily discusses SPME using absorptive polymeric coatings, although the principles also apply to adsorptive SPME onto active solid layers.
A related technique, stir-bar sorptive extraction (SBSE) uses a magnetic stir bar coated with a thick layer of absorptive polymer. The stir bar is exposed to sample solution for a time, during which solutes are absorbed into the polymer coating. Subsequently the stir bar is removed, dried, and then thermally desorbed for GC injection, or the absorbed analytes can be back-extracted with a different solvent. SBSE uses a larger volume of absorbent than SPME and, thus, is more efficient at extracting analytes with less absorbent solubility. Therefore, SBSE is generally more sensitive than SPME.
SPME applications cover a broad range that includes flower scents (3), chemical warfare agents (4), pharmaceutical process impurities (5), the determination of organochlorine pesticides in Chinese teas (6), volatile compounds in acidic media (7) and cheese (8), volatile phenols in wine (9), environmental pollutants in water samples (10), chloroanisoles in cork stoppers (11), volatile aliphatic amines in air (12), and phenylurea herbicides in aqueous samples (13).
SPME Principles
SPME relies on the extraction of solutes from a sample into an absorptive layer on the SPME fiber. After a sampling period — during which extraction ideally reaches equilibrium — the SPME fiber and captured solutes within its absorptive layer are transferred into an inlet system that then desorbs the solutes into a gas (for GC) or liquid (for LC) mobile phase. Success relies on choosing conditions so that the desired solutes favor the SPME absorptive layer as much as possible in the presence of bulk sample, and then subsequently the absorbed solutes are released as quickly and completely as possible for chromatographic analysis. Secondary trapping and release of desorbed solutes after SPME is sometimes required when the initial desorption is too slow and the resulting broadened peaks do not yield full utilization of column resolving power. This may be accomplished with a discrete thermal trap or with column stationary phase trapping by injecting onto a cold column and subsequently temperature programming for solute elution. It is also possible to make multiple SPME extractions and desorptions into a secondary trap for enhanced sensitivity.
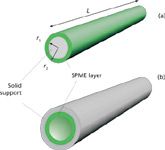
Figure 1: Cross-sectional diagram of SPME extraction devices. Shown are (a) a fiber device with external sorptive coating and (b) a tube device with sorptive coating on the inside.
For bulk samples contained in vials or otherwise easily accessed, SPME is performed conveniently with a short absorptive film–coated fiber, as shown in Figure 1a. A short tube coated on the inside with an absorptive layer (Figure 1b) may be used as well for samples that are amenable to pumping. The choice of the absorptive layer chemistry and film thickness strongly influences the degree of absorption and the subsequent efficiency of desorption. SPME fibers are available in syringe-integrated assemblies that are conveniently handled either manually or by robotic autosampling systems. Used fibers often can be cleaned up for reuse by solvent rinsing or baking.
Step 1: Extraction
For the extraction step with an externally coated SPME absorptive layer, the layer is exposed to sample in the liquid (Figure 2a) or gas (Figure 2b) phase. In the case of SPME within a coated tube, liquid or gaseous sample is circulated through the tube until equilibrium is reached. Stopped-flow flow-through sampling is also possible. Over time, the amounts of the solutes in the SPME layer reach an equilibrium level with their surroundings, which represents the maximum solute amounts that can be absorbed and withdrawn under a given set of sampling conditions. The amount of solute in the SPME layer at equilibrium (Mi,SPME) can be approximated by the following equation:
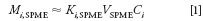
where Ki,SPME is an aggregate solute distribution constant between the SPME absorptive layer and the sample, VSPME is the volume of the SPME layer, and Ci is the solute concentration in the sample before performing SPME sampling.
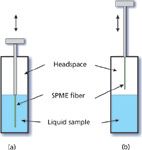
Figure 2: SPME extraction from a sealed vial. Shown are diagrams representing sampling (a) from the liquid phase and (b) from the headspace (gas phase).
Equation 1 assumes that the sample volume is much greater than the volume of the SPME layer. SPME coatings typically have thicknesses of about 10–100 µm — around 10 times the film thickness range normally encountered in capillary GC columns. The volume, V SPME, of a 1-cm long by 100-µm thick annular coating on a 0.56-mm o.d. (24-gauge) fiber (as shown in Figure 1a) is approximately 2 µL:
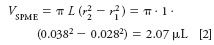
The volume of an SPME layer with the same thickness coated inside a tube (Figure 1b) with inner radius r would be the same. The assumption in equation 1 is valid for sample volumes of over 100 times the SPME layer volume, or more than about 0.2 mL for the thickest SPME layer of 100 µm. Thinner SPME layers, with smaller volumes, would imply correspondingly smaller minimum sample volumes.
Influence of the Headspace
The presence of a gaseous headspace over a liquid sample causes a portion of each solute to partition into the headspace in competition with the process of extraction into the SPME layer. This effect results in a reduction of solute mass in the SPME layer — relative to having no headspace present at all — that depends on both the headspace volume and the partition ratios between the headspace and the liquid sample as well as between the headspace or the liquid sample and the SPME layer. These relationships are somewhat complex and they produce dependencies of the extracted solute mass on the relative liquid and headspace volumes. From a practical point of view it becomes very important to maintain constant sample and headspace volumes across multiple samples to keep such multiphase influences consistent.
Time to Equilibrium
A finite time span is required to reach solute equilibrium between the sample and the SPME layer, which ideally will occur before the extracted solutes are withdrawn for desorption into a chromatograph. As solute molecules are removed from the sample into the SPME layer, additional solute molecules must diffuse into their place at the SPME–sample interface. The process of absorption is limited by the rate at which solute molecules can replenish the transition layer near the SPME interface. Stirring the liquid phase thoroughly helps reduce this time considerably by maximizing exposure of the SPME layer to the sample and greatly reducing the influence of solute liquid-diffusion rates on SPME uptake. However, stirring the liquid sample does nothing to increase the diffusion rate of absorbed solutes inside the SPME layer itself, which then becomes a limiting factor for the rate required to reach equilibrium. SBSE can benefit from decreased sorption times with rapid, turbulent stirring of the sample solution.
When sampling from the headspace gas instead of the liquid in a two-phase sample system, solute must first cross the liquid–gas interface before encountering the SPME layer. Interestingly, it makes little difference to the ultimate equilibrium solute amounts in the SPME layer whether the sample is obtained from the liquid or the gas. However, the time to equilibrium may be influenced strongly by the choice of the sampling phase. Nonpolar and volatile solutes that strongly favor the headspace phase will come to equilibrium more rapidly if the SPME layer is exposed to the headspace, and the solutes that favor the liquid phase will equilibrate more rapidly directly from the liquid phase.
Ultimately, chromatographers must characterize the equilibrium times for each solute of interest. If equilibrium is reached in a reasonable time period, perhaps less than 30 min, then they should use a sampling time at least that long. On the other hand, if an unreasonably long time is required, in terms of the time available for sampling and analysis, then it is possible to perform SPME without reaching equilibrium. In that case, the operator must ensure that the same SPME sampling time is used for each sample, and that time should be as long as possible.
The sample ionic strength, temperature, and other factors that influence the partition coefficients — both between the liquid sample and headspace and between the SPME layer and the liquid — also must be kept under careful control for good sample-to-sample consistency. Adding salt to an aqueous sample often will shift the partition coefficients for nonpolar solutes in favor of the SPME layer as well as speed up the time to attain equilibrium.
SPME Layer Chemistry
The chemistry of the sorptive SPME layer plays a significant role in enhancing or discriminating against classes of compounds. For the most part, SPME layers for GC absorb solutes in a manner related to their behavior as GC stationary phases: Polar SPME layers such as those containing polyesters or acrylates will enhance polar constituents and discriminate against nonpolar materials. Adsorptive layers with active carbon constituents will more strongly retain volatile components than layers made of nonpolar dimethylsilicones. However, some thought must be made in consideration of desorption as well, a very strongly held solute may prove too difficult to pry off the SPME layer for analysis. Nonpolar solvents may act to shift the effective polarity of the SPME layer as they are also absorbed, and changing the sample's solvent usually will influence the SPME behavior. This can be advantageous in method development, as a means to shift equilibrium as desired, but the solvent should remain fixed once selected.
Step 2: Transfer
The next step after sampling is to transfer the SPME layer and absorbed analytes away from sample exposure and into conditions for desorption into the chromatography mobile phase. For LC–SPME tube sampling with a multiposition valve connected to the SPME tube there is no need to physically move the SPME layer, but new solvating conditions must be established in place that promote solute desorption. An SPME fiber device, on the other hand, is removed from the sample container and transported over a distance to where the solutes are to be desorbed — the GC inlet or a solvent wash position. Removal from the sample environment immediately starts the absorbed solute concentrations shifting away from their in-sample values to lower levels as solutes naturally desorb into their surroundings. The rate of natural desorption is fairly low for many solutes, but volatile molecules may experience significant losses. In a laboratory situation, the transfer time from sample vial to instrument can be short enough so that losses are insignificant. Losses can be minimized during extended transport and storage by sealing the SPME layer into a small enclosure and then ensuring that the contents are included with the rest of the sample upon desorption. In addition to volatile sample losses, an SPME layer easily can pick up nonsample components from the ambient air, especially during extended transportation from remote sites. Enclosing the SPME layer will also prevent the influx of such contaminants. A number of commercially available SPME devices incorporate such a sealing system.
Step 3: Desorption
Once in place at a chromatograph, the SPME layer must then be exposed to conditions that cause the absorbed solutes to desorb with as close to 100% efficiency as possible, and in a sufficiently short time compatible with the chromatography mode in use. In the case of an SPME layer coated inside a tube, for LC analysis, a simple multiposition valve arrangement can switch from sample liquid flow to the mobile phase. Stopping the mobile-phase flow in the SPME tube allows time for solute desorption to come to equilibrium between the SPME layer and the liquid mobile phase before the desorbed materials are introduced into the column.
Desorption from fiber-based external SPME layers is more conveniently achieved by insertion into a standard GC capillary inlet system in much the same way as a syringe. For LC desorption and analysis, devices are available that wash the external SPME layer with mobile phase and pass the desorbed solutes into the LC injection loop.
Several trade-offs arise in the course of thermally desorbing an SPME layer for GC analysis. First, the desorption temperature must be high enough so that the solutes leave the SPME layer rapidly. A desorption that is too slow may lead to peak broadening and tailing unless additional arrangements are made for trapping solutes at the beginning of the GC column before temperature-programmed elution. Conversely, too high of an inlet temperature may induce thermal decomposition and introduce some contaminants into the column from septum bleed as well as from the SPME layer itself.
During sample desorption from an SPME fiber into a split–splitless inlet, the inlet split flow should be turned off so that all of the solutes may enter the column without splitting, as in splitless injection. It is unlikely that enough sample will be absorbed on an SPME layer to necessitate sample splitting. A narrow-bore inlet liner (often called a "splitless" liner) helps produce better peak shapes as well by limiting the volume into which the solutes may expand. After the SPME device has been withdrawn from an inlet splitter the split flow may be turned back on to purge the inlet of any remaining materials and prevent some degree of peak tailing.
Programmed-temperature vaporizer (PTV) inlet systems are well suited to SPME desorption because of their smaller internal volumes. For SPME use, they should be operated at the same elevated constant temperatures as conventional split–splitless inlets because PTV heat-up rates (on the order of a several hundred degrees per minute) may not be fast enough to produce sufficiently narrow peaks without some form of additional stationary-phase trapping.
Why SPME?
The primary advantages of SPME are its ability to decouple sampling from the matrix effects that would distort the apparent sample composition or disturb the chromatographic separation, its simplicity and ease of use, and its reduced or nonexistent solvent consumption. These characteristics combine to make SPME an attractive alternative to classical headspace or thermal desorption sampling, solid-phase extraction, and classical liquid–liquid extraction.
As with a number of related sample preparation and injection techniques such as headspace GC or thermal desorption, SPME lends itself well to handling difficult sample matrices, but with the added benefit of low cost and simplicity. SPME doesn't require elaborate and expensive instrument accessories for occasional use and yet seems to be capable of delivering very good manual results in the hands of skilled users. This cannot necessarily be said for manual headspace or thermal desorption sampling. Autosamplers are also available that perform repetitive unattended SPME sampling.
SPME does require careful optimization and consistent operating conditions for success, but this is true of related techniques as well. Any poorly characterized sampling technique has no valid use in the analytical laboratory, and the burden of developing an SPME method is no greater than for the other techniques. SPME has a significant place in the analyst's arsenal of sample preparation techniques.
References
(1) C.L. Arthur and J. Pawliszyn, Anal. Chem. 66, 2145–2148 (1990).
(2) C.L. Arthur, D.W. Potter, K.D. Buchholz, S. Motlagh, and J. Pawliszyn, LCGC North Am. 10(9), 656–661 (1992).
(3) P. Barták, P. Vednár, L. Cáp, L. Ondráková, and Z. Stránsky, JSS 26(8), 715–721 (2003).
(4) G.L. Hook, G. Kimm, G. Betsinger, P.B. Savage, A. Swift, T. Logan, and P.A. Smith, JSS 26(12–13), 1091–1096 (2003).
(5) R.P. Frost, M.S. Hussain, and A.R. Raghani, JSS 26(12–13), 1097–1103 (2003).
(6) L. Cai, J. Xing, L. Dong, and C. Wu, J. Chromatogr. A 1015(1–2), 11–21 (2003).
(7) W.A. Araújo, C.A. Lacerda, E.A. Cappelaro, and F.M. Lanças, JSS 26(6/7), 624–628 (2003).
(8) O. Pinho, C. Peres, and I.M.P.L.V.O. Ferreira, J. Chromatogr. A 1015(1–2), 23–30 (2003).
(9) R. Castro Mejas, R. Natera Marn, M.A. de Valme Garca Moreno, and C. Garca Barroso, J. Chromatogr. A 995(1–2), 11–20 (2003).
(10) H. Bagheri and A. Mohammadi, J. Chromatogr. A 1011(1–2), 1–9 (2003).
(11) F. Bianchi, M. Careri, A. Mangia, and M. Musci, JSS 26(5), 369–375 (2003).
(12) J. Namiesnik, A. Jastrzebska, and B. Zygmunt, J. Chromatogr. A 1016(1), 1–9 (2003).
(13) H.-H. Lin, Y.-H. Sung, and S.-D. Huang, J. Chromatogr. A 1012(1), 57–66 (2003).
John V. Hinshaw
"GC Connections" editor John V. Hinshaw is a Senior Scientist at BPL Global, Ltd., in Hillsboro, Oregon, and a member of LCGC's editorial advisory board. Direct correspondence about this column to the author via e-mail: lcgcedit@lcgcmag.com.

John Hinshaw

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









