Simultaneous Measurement of an API with its Counter-ion Using Corona CAD with HPLC-HILIC
The Application Notebook
The analysis of anions and cations is critical during drug development and related QC. Measure both the API and the counterion in a single run.
Nicholas Santiago, Anis H. Khimani, and Ian Acworth, ESA Biosciences, Inc.
The analysis of anions and cations is critical during drug development and related QC. Measure both the API and the counterion in a single run.
The analysis of anions and cations is critical during drug development and related quality control. In addition to selecting the appropriate counter ion during the formulation of an API, ion analysis also allows the identification of contaminating ions during the early stages of drug development. Ion-exchange chromatography (IC) with conductivity detection is used widely (1). However, it requires expensive instrumentation, consumables, two runs with different columns to measure anions or cations, and highly trained personnel.
A novel method using hydrophilic interaction chromatography (HILIC) (2) with UV was capable of simultaneously separating cations and anions but lacked sensitivity. HILIC with charged aerosol detection (CAD® ) allows simultaneous measurements in a single run with excellent sensitivity, consistent response, wide dynamic range, superior reproducibility, and ease of use (3). Here we show the simultaneously measurement of APIs and their counter ions, using conditions that are fully compatible with LC–MS analysis.
Materials and Methods
A standard HPLC system with a Corona Charged Aerosol Detector and a Shimadzu LC–MS 2010EV Mass Spectrometer.
HPLC Parameters:
Column: Sequant ZIC® -pHILIC 5 μm, 4.6 × 150 mm, 30°C
Mobile Phases: 75:25 acetonitrile (ACN)/100 mM ammonium acetate (pH 7.0); 85:15 ACN/150 mM ammonium acetate (pH 7.0); 60:40 ACN/25 mM ammonium acetate (pH 7.0); 75:25 ACN/75mM ammonium acetate pH 7.0
Mass spec: 5:1 flow split. Positive scan for procainamide and negative scan for diclofenac
Flow Rate: 1.0 mL/min, isocratic; Injection Volume: 10 μL
Results
The method was evaluated either using an API anion (diclofenac) and sodium counter ion, or an API cation (procainamide) and chloride counter ion. Figure 1 showed simultaneous analysis of the diclofenac and sodium counter ion. Changes in organic content (pH unchanged) markedly affected the retention time of sodium, while still resolving the API from the void [Figures 1a (sodium peak not shown on the chromatogram) and 1c]. Simultaneous confirmation (m/z) of the API was obtained by MS (Figure 1b). Similar results were obtained with procainamide and chloride counter ion analysis. Manipulation of mobile phase ionic strength and organic content (keeping the pH unaltered) actually inverted the retention order of procainamide and chloride counter ion (Figures 2a and 2c). Confirmation with MS identified the API (Figure 2b).
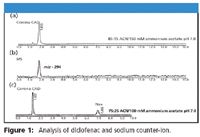
Figure 1
Conclusion
Simultaneous separation and identification in a single run was achieved with consistency and reproducibility. The identification of the active compound was confirmed by MS. The results clearly demonstrated the advantages of the HILIC-CAD for the simultaneous analysis of counter ions and API critical in drug development, therefore saving time, enhancing efficiency, and enabling detection at trace ng levels.

Figure 2
References
(1) Small, H. et al, W. Anal. Chem. 1975, 47, 1801–1809.
(2) Risley D.S.; Pack, B.W. LCGC North America 2006, 24, 776–785.
(3) "Is Ion Analysis Bringing You Down?" www.esainc.com/webinars.

ESA Biosciences, Inc.
22 Alpha Road, Chelmsford, MA 01824
Tel: (978) 250-7000, Fax: (978) 250-7087


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)















