Simultaneous Extraction and Quantification of Morphine-related Compounds in Plasma Using Mixed-mode SPE and UPLC–MS–MS
The Application Notebook
Zhe Yin, Kenneth J. Fountain, Erin E. Chambers and Diane M. Diehl, Waters Corporation, Milford, Massachusetts, USA.
Zhe Yin, Kenneth J. Fountain, Erin E. Chambers and Diane M. Diehl, Waters Corporation, Milford, Massachusetts, USA.
Introduction
Morphine is an effective pain-relieving drug that is primarily metabolized into morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G). The highly potent M6G may have adverse effects, such as respiratory depression and renal failure, if accumulated in the body. As morphine abuse continues to affect modern society, an effective method must be established to analyse morphine and its structurally related compounds in biological fluid samples. In this work, a UPLC–MS–MS method was developed to separate six morphine-related compounds on a 2.1 × 100 mm, 1.8 μm ACQUITY UPLC HSS T3 column in a single run using an ACQUITY UPLC system connected to a fast-scanning triple-quadrupole MS detector (TQD). The method achieved adequate retention of these very polar compounds by reversed-phase (RP) chromatography in an 8-min total run time.
Experimental

Results and Discussion
SPE recoveries for most of the compounds at 25 ng/mL were > 90% except for the glucuronide metabolites that had slightly lower recovery (Table 1). Inter-day SPE reproducibility was better than 6%. A representative chromatogram of a pre-extracted spiked sample is shown in Figure 1.
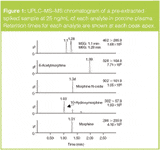
Figure 1
All calibration curves had an R2 ≥ 0.998 and % deviation for each point was < ± 15% of the expected values. The LLOQ values (five times the level in the blank matrix) for all analytes were determined to be 0.1 or 0.25 ng/mL.
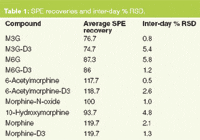
Table 1: SPE recoveries and inter-day % RSD.
Conclusion
A method for the simultaneous extraction and quantification of six morphine-related compounds in porcine plasma was developed. All six compounds were analysed in a single UPLC–MS–MS run in 8 minutes. A neutralizing collection step was used in the SPE protocol to prevent analyte degradation. The SPE procedure using an Oasis MCX μElution plate was able to achieve consistent recoveries ranging from 77% to 120%, depending on the analyte. The method was linear over at least 3 orders of magnitude with R2 ≥ 0.998 and achieved LLOQ values in the range of 0.1 to 0.25 ng/mL. This method achieves the desired detection limits in less time than previously published methods and addresses the issue of analyte instability for the selective extraction of polar compounds.
For the complete application note and results visit www.waters.com/28962
© 2009 Waters Corporation. Waters, The Science of What's Possible, UPLC, ACQUITY UPLC, Oasis and ACQUITY are trademarks of Waters Corporation.

Waters Corporation
34 Maple Street, Milford, Massachusetts 01757, USA
tel. +1 508 478 2000 fax +1 508 482 3605
Website: www.waters.com






















Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.
