A Simplified Method to Determine Total Sulphite Content in Food and Beverages via Ion Chromatography
The Column
Sulphite is a preservative added to a vast range of foods and beverages to prevent browning or oxidation. Some individuals are sensitive to sulphite additives and may experience a range of allergic reactions. Therefore, both the U.S. Food and Drug Administration (FDA) and European Union (EU) laws require that the presence of sulphites be declared on food labels when the concentration exceeds 10 mg/L. Several analytical methods exist to measure sulphite in food and beverages, however, they suffer from repeatability issues, and can be quite cumbersome to perform. A patent has been filed for an innovative, fast, and accurate ion chromatographic (IC) method based on direct current (DC) mode electrochemical detection. This article explains more.
Photo Credit: Aliaksei/stock.adobe.com

Sulphite is a preservative added to a vast range of foods and beverages to prevent browning or oxidation. Some individuals are sensitive to sulphite additives and may experience a range of allergic reactions. Therefore, both the U.S. Food and Drug Administration (FDA) and European Union (EU) laws require that the presence of sulphites be declared on food labels when the concentration exceeds 10 mg/L. Several analytical methods exist to measure sulphite in food and beverages, however, they suffer from repeatability issues, and can be quite cumbersome to perform. A patent has been filed for an innovative, fast, and accurate ion chromatographic (IC) method based on direct current (DC) mode electrochemical detection. This article explains more.
Sulphite (SO32-) is a widely used preservative added to prevent browning or oxidation to a vast range of food and beverage products including meat, fish, legumes, soft drinks, wine, and beer (1,2). Sulphite is also a naturally occurring substance produced during fermentation processes (2). Generally speaking, sulphite is an oxygen scavenger, resulting in formation of either sulphate (SO42-) or sulphur dioxide (SO2) after the oxidation process, depending on the conditions (3).
Many individuals are sensitive to sulphite additives and some have experienced mild to severe allergic reactions, however, the exact mechanism behind this remains unknown, despite several hypotheses tested. Therefore, both U.S. Food and Drug Administration (FDA) and European Union (EU) laws require that the presence of sulphites be declared on food labels when the sulphite concentration is higher than 10 mg/L (4). For certain people, sulphite exposure in food has been reported to induce asthmatic episodes (5), though further tests have been performed to determine how this affects the asthmatic population specifically, as this sensitivity may have been overestimated in previous studies (6).
Methods of Sulphite Measurement in Foodstuffs
Traditionally, the optimized MonierâWilliams (OMW) Official Method 990.28 was used for quantification of sulphite in most matrices (7), but this method is time-consuming, labourâintensive, and has a method detection limit (MDL) at the regulatory labelling threshold. More efficient and accurate methods have been tested in the meantime using stabilizers to overcome sulphite chemical instability in aqueous solution followed by different detection techniques. Such techniques are based on ion chromatography (IC) with conductivity detection (8,9), as well as chromatographic methods based on ionâexclusion chromatography with DC mode electrochemical detection (10), and ion-exclusion chromatography with pulsed amperometric detection (PAD) (11).
Automated discrete analysis methods have been reported for sulphite analysis (12). These methods mainly focus the sulphite analysis on specific sample matrices such as wine, beer, dried fruits, and so on. Nevertheless, laboratories where sulphite analysis is required for a wide variety of food and beverage products need a single, robust analytical method to provide a solution. Methods based on IC with conductivity detection offer a lack of selectivity combined with an extended analysis time due to the need to separate sulphite from sulphate, as well as
from many other coeluting organic interferents.
A newer method developed by AOAC (Method 990.31) shifted away from gravimetric titration to focus on the use of ion-exclusion chromatography followed by electrochemical (amperometric) detection of samples after extraction in an alkaline medium (10). However, AOAC Method 990.31 is not applicable to dark-coloured foods or ingredients because the sulphite is so strongly bound. The added sulphite in food products can bind irreversibly to the aldehyde groups in sugars, and therefore the preservative effect is reduced, especially in dark foods where the Maillard reaction has taken place (13). Some types of caramel coloured food additives are made with sulphites (Caramel Colour II / Caustic Sulphite Caramel, and Caramel Colour IV / Sulphite Ammonia Caramel) (13,14), which can cause problems for this method. Another issue involves the sensitivity of the detector. After a few injections, fouling from contaminants rapidly decreases the electrode sensitivity. Frequent reconditioning of the working electrode is necessary due to a rising background and baseline noise, and can be accomplished in a couple of ways. Manual polishing and utilizing PAD pulse sequences are the most common choices to recondition the surface of the working electrode, while other methods opt for disposable electrodes to avoid this step altogether (11).
Sulphite Analysis, Simplified
This article describes an innovative, fast, and robust IC method for the determination of total sulphite in food and beverage samples based on electrochemical detection (DC mode). A range of food and beverage products were analyzed, with sulphite recovery values near 100% in all cases under the defined method conditions. Using a single, robust chromatographic method, samples can be treated identically, saving time and making laboratory work much easier.
Background: Sulphite analysis is a necessity for foods and beverages to be compliant with various food labelling regulations. Most foods and beverages contain residual sulphite concentrations within a range of approximately 10–2000 mg/kg. For this article, a request arrived from two different laboratories with the goal of measuring total sulphite in all types of foodstuffs within 10 min per sample. The first laboratory was using high performance liquid chromatography (HPLC) followed by conductivity detection, where interferences from organics created several challenges. This method showed both a lack of selectivity and long analysis times due to the need to separate sulphite from sulphate, as well as from coeluting organic interferences. The second laboratory was using the optimized Monier-Williams (OMW) Official Method 990.28 (7). Long and tedious sample preparation meant that two analysts could complete only five sample measurements per day. Other analytical methods also involve demanding sample handling steps, with results disadvantaged by sensitivity and recovery issues. Therefore, a robust, selective, sensitive, and fast determination method was needed to overcome the drawbacks of conventional procedures.
Method
The general analytical procedure developed consists of an alkaline extraction of the sample, then anion exchange separation via IC using a high-capacity stationary phase and a high-strength sodium hydroxide–sodium acetate eluent, followed by DC mode electrochemical detection. Working electrode reconditioning was performed fully automatically after each analysis using a combination of techniques. A recovered electrode surface is automatically available at the beginning of every analysis, which is a first for LC/HPLC analysis of sulphite in these types of samples.
Several foodstuff matrices (both solid and liquid) were tested against this method including chickpeas, mustard, cherries, capers, canned garlic, chili pepper, and red wine. Each sample was measured via IC after dilution with a stabilization solution (Table 1) in a 1:30 ratio (w/w), as well as spiked with a known amount of sulphite standard to determine recovery. Automatic integration using peak areas was evaluated with software.
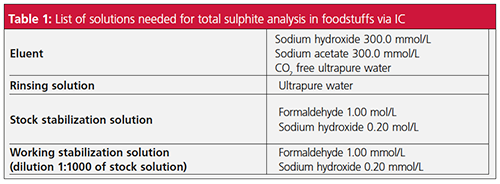
Stabilization Solutions: Sulphite is a highly unstable ion in solution. To prevent rapid oxidation to sulphate, it is always necessary to work with a stabilization solution. Fresh solutions must be prepared daily in order to achieve the most accurate and reproducible results. The ultrapure water must be degassed prior to eluent preparation as well as for all other working solutions and standards. The stabilization stock solution can be prepared from commercial reagents, and then diluted 1:1000 with degassed ultrapure water to obtain a working stabilization solution (Table 1).
Sample Preparation: All samples and standards were prepared with fresh working stabilization solution. After preparing the fresh stabilization solutions and preparing the sample extracts, samples were then passed through a 0.2 µm filter to prevent bacteria from influencing the results before completely filling 2 mL sample vials covered with a septum.
Solid Samples:
Solid samples should be processed by chopping and crushing until homogenized. A 1 g portion of sample was measured into a tared vortex tube, then the diluted stabilization solution was added until the total weight equaled 30 g (sample dilution 1:30 w/w). After covering, the solution was blended in the tube for 1 min using an Ultra-Turrax homogenizer from IKA (or similar equipment), then mixed manually with a vortex shaker or automatically with high-throughput plant and animal tissue homogenizer. Approximately 3 mL of the supernatant was aspirated from below the lipid layer, and the sample manually passed through a 0.2 µm filter into a 2 mL HPLC sample vial. The sample vial was completely filled to avoid leaving any headspace, which could lead to sulphite oxidation.
Liquid Samples:
A 1 g aliquot of sample was measured into a tared vortex tube, then the diluted stabilization solution was added until the total weight equaled 30 g (sample dilution 1:30 w/w). The solution was mixed manually with a vortex shaker for 15 min after covering the tube. Approximately 3 mL of the supernatant was aspirated and the sample was manually passed through a 0.2 µm filter into a 2 mL HPLC sample vial. The sample vial was completely filled to prevent further sulphite oxidation from the headspace.
Analysis: Eluent and stabilization solutions were prepared from degassed ultrapure water directly before launching the analytical sequence (Table 1). The sample exposure to air was minimized to prevent (further) sulphite oxidation. This includes covering the completely filled sample vials with a septum, which leads to higher recovery values and better reproducibility of the results. For longer sample series, it is recommended to use an autosampler that includes a cooling function, as this can extend the stability of the prepared samples to at least 24 h.
Measurements were performed on an ion chromatograph system (Metrohm 930 Compact IC Flex) with a high capacity analytical column (Metrosep Carb 2 - 150/4.0 with a Metrosep Carb 2 Guard/4.0 to protect the column [both Metrohm]) housed in a column oven to ensure a constant temperature for all analyses. An amperometric detector, utilizing a reference electrode (Ag/AgCl) and working electrode (Au), was utilized in DC mode for sulphite measurement. The detector was set to the same temperature as the column oven for the most accurate results.
Analysis Conditions for the Measurement of Total Sulphite via IC:
Eluent flow: 0.5 mL/min; run time: 8 min; reconditioning of electrode: 2 min; detector mode: DC; channel measurement: current (nA); E(V): 300 mV; range: auto; damping: off; detector temperature: 35 °C; column oven temperature: 35 °C; sample loop (partial loop mode): 3 µL; sample cooling temperature: 6 °C.
After each chromatographic determination, the working electrode surface was fully reconditioned within 2 min. No manual intervention was necessary, and there is no need to buy disposable electrodes. In this way, each analysis was performed in exactly the same manner, making this a robust, time-saving method with excellent reproducibility of results.
Results
Any kind of sample can be measured with this method without needing to change the parameters. With the newly developed electrode reconditioning step, a fresh working electrode surface is available after only 2 min, which fulfills the customer request of a complete total sulphite analysis within 10 min (including 8 min per chromatogram run).
It is important to remark that the combination of a very high capacity column and the eluent revealed an important improvement of peak resolution between formaldehyde and sulphite, reducing sample matrix effects as well as exhibiting very precise repeatability of retention times.
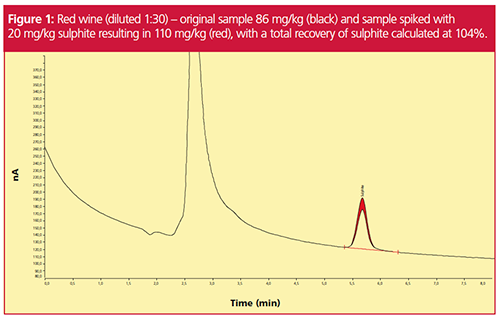
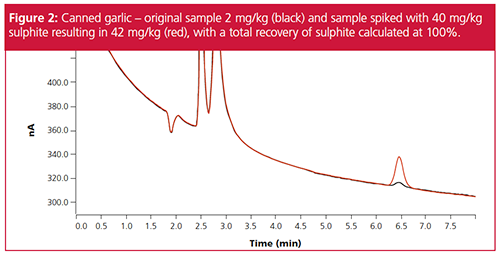
Not only is this method more robust than previous methods, it is also extremely sensitive to sulphite, as can be seen in Figure 1 and Figure 2. Retention times for these figures differ because the samples were analyzed in two separate laboratories with different setups.
Conclusions
This fast and accurate IC method overcomes several limitations encountered in total sulphite analysis based on the different analytical methodologies used to date and is valid for any kind of food or beverage products. The simplified sample preparation technique allows both liquid and solid foodstuff to be analyzed with the same method. Since the retention time for sulphite never changes with this method configuration, a range of samples can be analyzed concurrently without the need to modify anything.
A newly developed electrode treatment procedure enables this method to run quickly and efficiently for all kinds of complex matrices without fouling of the electrode surface, which would lead to nonârepeatable, unreliable results. Utilization of an autosampler with cooling function extends the stability of prepared food samples to allow uninterrupted total sulphite determination for a full 24 h. With a complete analysis time of 10 min per sample, including the automatic reconditioning of the working electrode, this method offers the option of measuring total sulphite in up to 144 samples per day. This result is a vast improvement upon the current sample throughput of five samples per shift (or 15 samples every 24 h) at the contract laboratory, who were behind the original request to develop a new method for total sulphite analysis in foodstuffs.
References
- B. Wedzicha, Chemistry of Sulphur Dioxide in Foods (Elsevier Applied Science Publishers, Barking, UK, 1984).
- S. Taylor, N. Higley, and R. Bush, Advances in Food Research30, 1–76 (1986).
- S. Taylor, R. Bush, and J. Nordlee, in Food Allergy: Adverse Reactions to Foods and Food Additives, D. Metcalfe, H. Sampson, and R. Simon, Eds. (John Wiley & Sons, Chichester, UK, 5th ed., 2013) pp. 361–374.
- US Food and Drug Administration, CFR Title 21, Volume 2, Section 130.9: Sulfites in standardized food (FDA, Silver Spring, Maryland, USA, 2019). Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=130.9
- R. Simon, Pediatric Allergy and Immunology3(4), 218–221 (1992).
- H.A. Vally, Thorax56, 763–769 (2001).
- AOAC International, AOAC Official Method 990.28, Sulfites in Foods, Optimized MonierâWilliams Method (AOAC International, Rockville, Maryland, USA, 1994).
- J. Lin, Y. Zhu, W. Cheng, J. Wang, B. Wu, and J. Wang, Food Science and Technology Research20(5), 1079–1085 (2014).
- B.S. Liao, J.C. Sram, and D.J. Files, Journal of AOAC International 96(5), 1103–1108 (2013).
- AOAC International, AOAC Official Method 990.31, Sulfites in Foods and Beverages, Ion Exclusion Chromatographic Method (AOAC International, Rockville, Maryland, USA, 1995).
- L. Chen, B. De Borba, and J. Rohrer, Determination of Total and Free Sulfite in Foods and Beverages (Application Note 54), Thermo Fisher Scientific, Sunnyvale, California, USA (2016).
- E. Naigeon, M. Rastetter, M. Klemm, and A. Suoniemi-Kähärä, Fast and Accurate Automated Method for Free Sulfite Analysis in Wine (Application Note 71451), Thermo Fisher Scientific, Sunnyvale, California, USA (2014).
- T.A. Vollmuth, Food and Chemical Toxicology111, 578–596 (2018).
- P.R. Ashurst, R. Hargitt, and F. Palmer, Soft Drink and Fruit Juice Problems Solved (Woodhead Publishing, UK, 2nd ed., 2017), pp. 67–94.
Alyson Lanciki is currently the Scientific Editor of Metrohm AG, a manufacturer of high-precision instruments for laboratory and process analysis. After a postdoctoral position in France, Alyson joined the Metrohm family in 2013 as an ion chromatography application specialist in the Netherlands at Metrohm Applikon, where she eventually became the global marketing manager for the Metrohm Process Analytics brand of instruments.
Miguel Espinosa is currently the Product Manager for Ion Chromatography at Metrohm Hispania, located in Madrid, Spain. Miguel joined Metrohm in 1999 as an application specialist for ion chromatography.
E-mail:info@metrohm.com / ic@metrohm.esWebsite:www.metrohm.com/es-es

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.













