Chromatographic Media Selection and Optimization for Hybrid LBA/LC–MS/MS Workflows
The Column
This article describes the importance of magnetic bead and chromatographic media selection for hybrid LBA/LC–MS/MS workflows.
Zffoto/stock.adobe.com

Traditional ligand binding assays (LBA) as a method for quantification of biotherapeutics in biologics matrices are being coupled with liquid chromatography tandem mass spectrometry (LC–MS/MS) to obtain more specificity and increase linear dynamic range (LDR), thus creating hybrid LBA/LC–MS/MS methods. This hybrid workflow is more sensitive and consistent than traditional methods, which is critical in efficacy and safety testing. Magnetic beads help to streamline the LBA portion of the protocol, which replaces enzyme-linked immunosorbent assays (ELISA). Additionally, chromatography optimization for the LC–MS/MS step can ensure a robust analysis. This article describes the importance of magnetic bead and chromatographic media selection for hybrid LBA/LC–MS/MS workflows.
During pre-clinical and clinical studies, researchers must establish the pharmacokinetic (PK) properties of biotherapeutics. The primary bioanalytical technique for large molecule quantitation historically utilized ligand binding assays (LBA), specifically enzyme-linked immunosorbent assays (ELISA). While still the most employed technique for immunoassays, the shortcomings of ELISA methods, such as unreliable results and lengthy protocols, prompted researchers to seek alternative methods to accurately extract and quantitate biotherapeutics from biologic matrices. Mass spectrometry (MS)-based techniques provide more accurate results and give more detailed PK information than LBA methods (1,2). Employing these processes together, deemed hybrid LBA/liquid chromatography (LC)–MS/MS (3–5), allows for the development of a platform approach to establish PK properties of biotherapeutics, currently a challenge with ELISA alone (6,7). The chromatographic separation during the LC–MS/MS protocol provides additional intriguing components and this article will demonstrate how the optimization of monoclonal antibody (mAb) quantitation from biological matrices further leads to a streamlined LBA/LC–MS/MS protocol.
Materials and Methods
Rituximab and trastuzumab were purchased from Myoderm. Trypsin was purchased from Promega Corporation. SiLuMab and Dulbecco’s Phosphate Buffered Saline (DPBS) were purchased from SigmaâAldrich. bioZen MagBeads, a 150 × 2.1 mm bioZen Peptide XB-C18 column, and a 150 × 2.1 mm bioZen Peptide PS-C18 column are from Phenomenex. LC–MS/MS methods were performed on an Agilent 1290 system equipped with a Sciex X500B QTOF.
Immunocapture Protocol: Per the sample prepared, 20 µg of streptavidinâcoated MagBeads were incubated with 1 µg of biotinylated goat anti-human IgG. After 1 h incubation, the beads were washed with PBS using a magnetic stand. Immunocapture beads were then incubated with rat serum spiked with a range of concentrations of target mAb and internal standard (SILuMab). Supernatant was discarded and the captured mAbs were eluted with 0.1% TFA.
Trypsin Digestion Protocol: The eluted samples were diluted with ammonium bicarbonate, ensuring pH >7.0. Samples were heat denatured to 95 °C. After cooling to <50 °C, trypsin with 0.1% formic acid was added, and the mixture was shaken at 300 RPM for 1 h at 50 °C. After centrifugation, the sample was analyzed by LC–MS/MS.
Results and Discussion
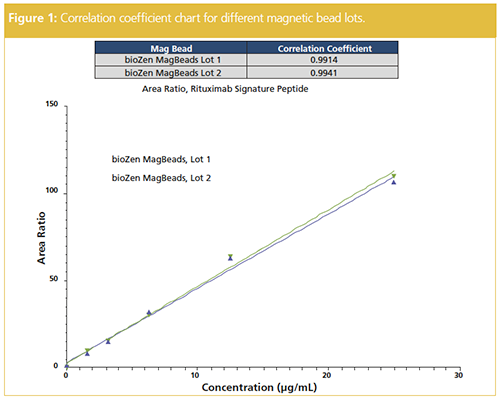
To ensure consistent immunocapture, two different lots of the magnetic beads were assessed (Figure 1). The correlation coefficients were greater than 0.99 for both lots providing confidence in the LBA protocol. This lot-to-lot reproducibility assessment is important to ensure consistency in biotinylated capture antibody, leading to potential variation in linear dynamic range (LDR) of the assay.
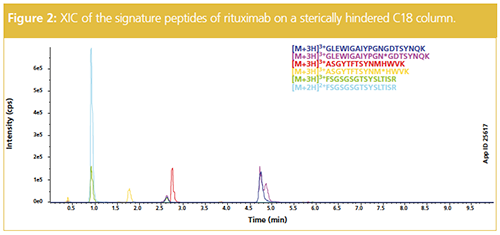
For PK studies, signature peptides with high ionization potential and predictable fragmentation patterns are selected for LC–MS/MS analysis. Additionally, signature peptides must maintain their integrity throughout the sample preparation and MS analysis. Moreover, achieving optimal chromatographic retention and separation of these peptides is ideal for LC–MS/MS analysis because a peptide with more retention will typically result in better ionization. Optimal peak shape ensures correct quantitation and improves LDR. Finally, chromatographic selectivity of the chosen peptides is necessary and depends on the number of peptides being quantitated. Considering these factors, Figure 2 shows the analysis of the signature peptides of rituximab using a C18 column. The six signature peptides from digested rituximab were quantitated with excellent recovery and sensitivity at 1 µg injection.
A critical aspect of the LC–MS/MS separation of digested peptides is the column selection. This includes both surface chemistry, which can impact peak shape and selectivity, as well as particle morphology, which can result in peak capacity-a measure of efficiency when running a chromatographic separation in gradient mode.
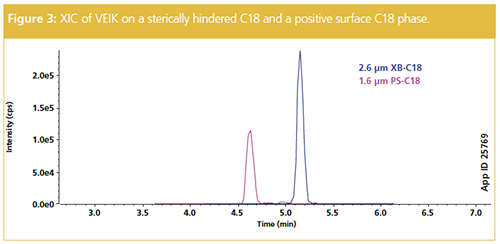
For most peptide quant applications, a standard C18 chemistry is used, but not all C18 phases provide the equivalent performance. Although generally providing higher peak capacities and superior peak shapes, C18 phases with a positive surface charge are overall less retentive than a nonâcharged surface. Conversely, a stericallyâhindered C18 may increase retention and this can assist in sensitivity for particularly polar peptides. Figure 3 shows the extracted ion chromatogram (XIC) for signature peptide VEIK from a trastuzumab digest using two different C18 phases. The positive surface C18 is less retentive and elutes VEIK earlier than the more retentive sterically-hindered C18 phase. Therefore, an optimal column media should be selected once the hydrophobicity of each mAb signature peptide is assessed.
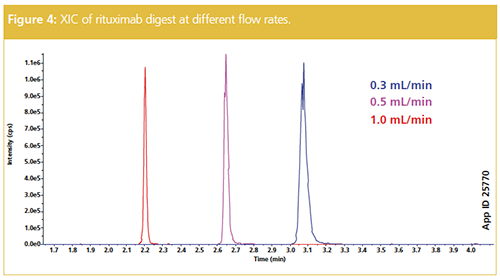
One other factor for column media selection is the particle morphology, particularly if sample throughput is of concern. Utilizing a superficially porous 2.6 µm core–shell particle provides similar peak capacity to a sub-2-µm fully porous particle. However, because the 2.6 µm particle does not generate as high a back pressure, higher flow rates might be implemented. Figure 4 shows the increase in flow rate from 0.3 mL/min to 1.0 mL/min of a rituximab digest. The peak capacity increases with the higher flow rate and allows a more rapid assessment of signature peptides. It is important to note that the higher flow rates are not at the expense of sensitivity; only a nominal decrease in counts per second (cps) is observed in the example (Figure 4).
Conclusion
As hybrid LBA/LC–MS/MS methods gain more traction in the industry, a thorough assessment of each method parameter is important for method optimization. This article has demonstrated the importance of lot-to-lot reproducibility of magnetic beads for LBA as well as column selection for
LC–MS/MS. In the results presented,
core–shell particles with a sterically hindered C18 provided the best results for the mAbs selected; however, as mAbs vary in composition and biotherapeutics become more complex, assessment of appropriate column media should not be overlooked. Optimizing these components will increase confidence in platform methods in streamlined PK studies.
References
- C.W. Damen, J.H.M. Schellens and J.H. Beijnen, Human Antibodies18(3), 47–73 (2009).
- E. Ezan and F. Bitsch, Bioanalysis1(8), 1375–1388 (2009).
- M. Yuan, O.A. Ismaiel, and W.R. Mylott Jr, Reviews in Separation Sciences1(1), 47–55 (2019).
- S. Ramagiri and I. Moore, Bioanalysis8(6), 483–486 (2016).
- K. Xu, L. Liu, M. Maia, J. Li, J. Lowe, A. Song, and S. Kaur, Bioanalysis6(13), 1781–1794 (2014).
- Y. Zhang, T. Olah, and J Zeng, Bioanalysis6(13), 1827–1841 (2014).
- A. Liu, A. Kozhich, D. Passmore, H. Gu, R. Wong, F. Zambito, V.S. Rangan, H. Myler, A.-F. Aubry, M.E. Arnold, and J. Wang, Journal of Chromatography B1002, 54–62 (2015).
Christina Malinao received her B.S. in neuroscience and biology from the University of California, Riverside (USA). She spent some time at Schering Plough performing biomarker characterization, and several years at Agensys performing antibody and antibody–drug conjugate characterization. She joined Phenomenex as a scientist in the Phenologix application laboratory performing method development. Christina currently works at Tae Life Sciences.
Brian Rivera is the Senior Product Manager – Biologics at Phenomenex. He has worked at Phenomenex for nine years, holding other positions including technical support and sales. Prior to joining Phenomenex, Brian worked within the biotechnology industry, including positions focused on protein purification, analytical method development, and in-process analytical support. Brian has a bachelor’s degree from the University of California, Davis, USA.
Chad Eichman is the Global Business Unit Manager – Biopharmaceuticals at Phenomenex, Inc. where he develops and leads the strategic plans for Phenomenex’s biopharmaceutical business. He received his B.S. in chemistry from the University of Wisconsin-Madison (USA) and his Ph.D. in organic chemistry from The Ohio State University (USA). After a postdoctoral appointment at Northwestern University (USA) and an assistant professorship at Loyola University Chicago (USA), Chad joined Phenomenex in 2017.
E-mail: ChadE@phenomenex.comWebsite: www.phenomenex.com/bioZen

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.











