Postnova - Separation of Nanoplastics and Determination of Their Surface Charge by Electrical Asymmetrical Flow Field-Flow Fractionation
Plastic micro- and nanoparticles are increasingly in the headlines, particularly when discussing marine pollution, but also with regard to their potential impact on human health. In this application note, we present data on separation of polystyrene nanoplastics, and demonstrate how electrical asymmetrical flow field-flow fractionation (EAF4) can be used for simultaneous size separation and particle surface charge measurement.
Plastic micro- and nanoparticles are increasingly in the headlines, particularly when discussing marine pollution (1), but also with regard to their potential impact on human health (2). Typically formed by the weathering and breakdown of plastic materials in the environment, nanoplastics are challenging to separate and characterize by commonly used techniques such as dynamic light scattering or size exclusion chromatography. In this application note, we present data on separation of polystyrene nanoplastics, and demonstrate how electrical asymmetrical flow field-flow fractionation (EAF4) can be used for simultaneous size separation and particle surface charge measurement. A schematic for the EAF4 channel and its separation principle is shown in Figure 1.
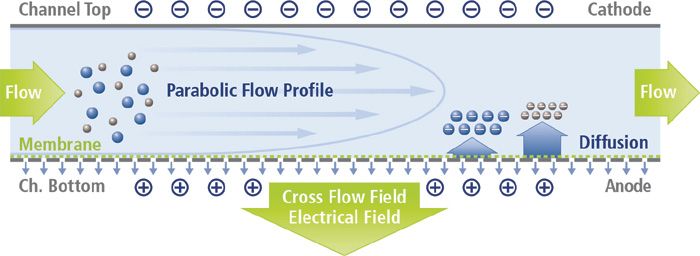
Figure 1: Schematic representation of an electrical asymmetrical flow field-flow fractionation channel.
Experimental Details and Results
A mixture of two polystyrene latex particles (nominal diameters of 61 nm and 125 nm, respectively) was used as a proxy for a polydisperse nanoplastics system. This mixture was separated by EAF4 using four different electrical field conditions, enabling measurement of the electrophoretic mobility and thus the surface zeta potential of both particles. In addition, multi-angle light scattering (MALS) was used as a detector to simultaneously collect information about the size of both particles.
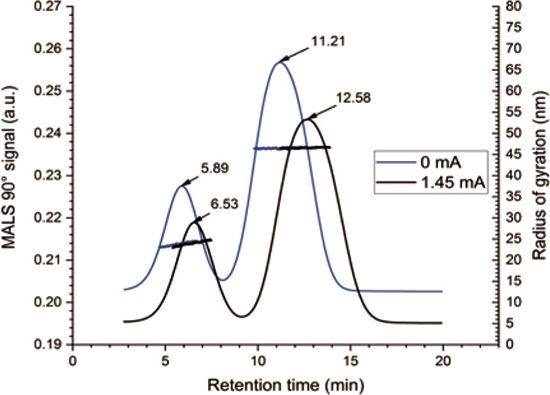
Figure 2: EAF4-MALS fractograms of the investigated nanoplastics particle mixture with and without application of an electrical field (black and blue graph, respectively). The blue and black dotted lines display the radii of gyration obtained from MALS indicating no influence of the electrical fi eld on the particle size.
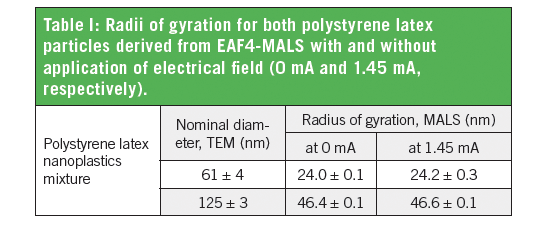
Figure 2 displays two EAF4-MALS fractograms. In the first fractogram (blue graph), separation was achieved solely by the cross flow field without application of an electrical field (0 mA), while, in the second fractogram (black graph), an additional electrical field (1.45 mA) was applied. It can be clearly seen that the electrical field induced a measurable shift in the retention time due to the surface charge of both particles. At the same time, the measured size of both particles (radius of gyration, Rg, blue and black dotted line) remained unaffected, highlighting that there is no influence of the electrical field on the stability of the particle mixture (Table I).
In order to derive reliable data about the electrophoretic mobiliy and zeta potential of a sample, repeated EAF4 measurements under similar cross flow conditions, but different electrical fields, needed to be performed.
Figure 3 displays the EAF4-MALS fractograms of the investigated nanoplastics mixture obtained under four different electrical field strengths. By measuring the shift in retention time and relating it to the applied electrical field, the electrophoretic mobility and zeta potential of the particles can be calculated.
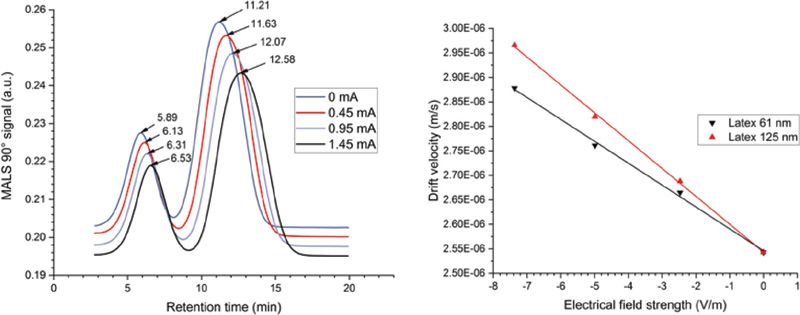
Figure 3: EAF4-MALS fractograms of the investigated nanoplastics particle mixture obtained for four different electrical field strengths (left). Differential velocity versus electrical field strength plot to determine the electrophoretic mobility of the two nanoplastic particles in the mixture (right).
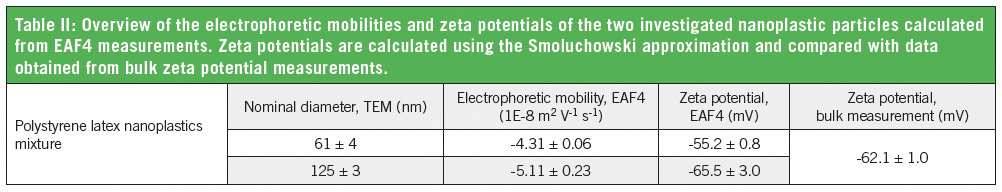
Comparing the EAF4 results with data obtained from bulk zeta potential measurements clearly highlight the advantage of EAF4 for polydisperse samples, particularly when sample constituents exhibit different surface charges (Table II).
Conclusion
The EAF4 system allows both size and surface charge separation, enabling determination of size or molecular weight distribution and electrophoretic mobility and zeta potential in one single instrument. As applications for nanoplastics analysis increase, high-resolution separation techniques will be required for these likely polydisperse distributions. Different polymer materials may have different electrophoretic mobility, leading to the need for a characterization tool such as EAF4 to provide size and charge information for complex samples.
References
(1) L.M. Rios Mendoza, H. Karapanagioti, N.R. Alvarez, Curr. Opin. Environ. Sci. Health, 1, 47–51 (2018).
(2) M. Hesler, L. Aengenheister, B. Ellinger, R. Drexel, S. Straskraba, C. Jost, S. Wagner, F. Meier, H. von Briesen, C. Büchel, P. Wick, T. Buerki-Turnherr, and Y. Kohl, Toxicol. in Vitro, 2019, in press.

Postnova Analytics Inc.
Salt Lake City, UT 84102, USA
tel. +1 801 521 2004
Website: www.postnova.com

Separation of Ultra-Short and Long Chain PFAS Compounds Using a Positive Charge Surface Column
December 11th 2024A separation of ultra-short and long chain PFAS (C1-C18) is performed on a HALO®PCS Phenyl-Hexyl column along with a HALO®PFAS Delay column which demonstrates excellent retention for both hydrophilic and hydrophobic analytes.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










