Macherey-Nagel - Determination of Triphenylmethane Dyes from Aquaculture Samples
The Application Notebook
This application note describes the determination of the triphenylmethane dyes Malachite Green and Crystal Violet and their metabolites from the aquaculture samples brown trout, shrimp, and tuna using dispersive SPE (dSPE) with CHROMABOND QuEChERS mixes for sample clean-up.
This application note describes the determination of the triphenylmethane dyes Malachite Green and Crystal Violet and their metabolites from the aquaculture samples brown trout, shrimp, and tuna using dispersive SPE (dSPE) with CHROMABOND® QuEChERS mixes for sample clean-up. The eluates from dSPE cleaning are finally analyzed by HPLC–MS/MS using the biphenyl phase NUCLEODUR® π2.
Motivated by various potential health benefits, human feeding behaviour is changing, leading to an increasing demand for aquatic products. Farming aquatic species is becoming an important way of producing large amounts of aquatic products all over the world. One challenge of farming aquatic species is the control of infectious diseases.
Triphenylmethane dyes (TPM) are organic compounds originally used as textile and paper dyes and have been found to be effective as antibacterial, antifungal, and antiparasitic agents in fisheries. They accumulate in fish and metabolize to the equivalent, colourless leuco-forms, which are also known as mutagenic (1). To protect human health, triphenylmethane dyes have been banned in many countries according to the recommendations of national and international related agencies. For example, the European Union has implemented a minimum required performance limit (MRPL) for the sum of Malachite Green and Leucomalachite Green of 2 μg/kg (2).
QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe) methodology is known as a common sample preparation technique in modern analysis of pesticides in food, and offers a quick and cost-efficient determination of different analytes in strongly matrixâcontaminated samples by GC–MS and LC–MS (3).
Biphenyl-modified HPLC phases feature a better retention of aromatic analytes compared to classical C18 phases due to additional π-π interactions.
Dispersive Solid-Phase Extraction (4)
Extraction:
- Weigh out 5 g of homogenized sample into a 50 mL brown centrifuge tube
- Add 5 μL of internal standard solution (β = 1 μg/mL Malachite Green-d5 and Leucomalachite Green-d5 each in acetonitrile)
- Add 25 μL of standard solution (β = 200 ng/mL Malachite Green, Leucomalachite Green, Crystal Violet, Leucocrystal Violet each in acetonitrile) for determining recovery rate
- Add 5 mL water and shake
- Add 10 mL 1% acetic acid in acetonitrile and shake
- Add the CHROMABOND® QuEChERS extraction Mix II (MachereyâNagel REF 730971)
- Shake vigorously for 30 s and cool the mixture down in an ice bath
- Centrifuge the mixture at 4500 rpm for 5 min at 4 °C
Clean-up:
- Put 5 mL acetonitrile supernatant in a 15 mL brown centrifuge tube
- Add the CHROMABOND® QuEChERS clean-up Mix III (MachereyâNagel REF 730972) (for samples with high fat content add 1 mL hexane)
- Shake vigorously for 30 s
- Centrifuge the mixture at 4500 rpm for 5 min at 4 °C
- Dilute the extract 1:1 with an aqueous solution of 5 mmol/L ammonium acetate + 1 mL/L formic acid and filter through a syringe filter (CHROMAFIL® Xtra PET-20/13, Macherey-Nagel REF 729208)
Subsequent Analysis: HPLC–MS/MS (5)
HPLC column: EC 100/3 NUCLEODUR® π2, 3 µm (Macherey-Nagel REF 760636.30)
Eluent A: 5 mmol/L ammonium acetate + 0.1% formic acid in water
Eluent B: acetonitrile
Gradient: 20–85% B in 10 min, 85% B for 5 min, 85–20% B in 1 min, 20% B for 5 min
Flow rate: 0.4 mL/min
Temperature: 25 °C
Injection volume: 5 µL
MS/MS detection: LCMS 8050 (Shimadzu); ion source: ESI, positive ionization mode; scan type: MRM; interface heater on; interface current: 4000 V; interface temperature: 300 °C; DL temperature: 250 °C; nebulizing gas flow: 3.00 L/min; heating gas on; heating gas flow: 10.00 L/min; heat block: 400 °C; drying gas on; drying gas flow: 10.00 L/min
Results
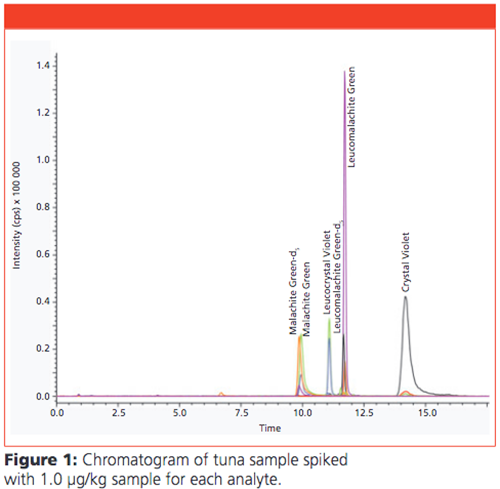
The identification and quantification of TPM dyes in the cleaned sample extracts of brown trout, shrimp, and tuna were successfully performed by ESI mass spectrometry. Calibration curves were in the concentration range between 0.5 ng/mL and 50 ng/mL for each analyte. Figure 1 shows the chromatogram of QuEChERS extract from the tuna sample spiked with 1 µg/kg with high signal response and less matrix interferences.
By using the QuEChERS methodology it was possible to recover more than 70% of TPM dyes from the different matrices (see Figure 2). The use of brown centrifuge tubes protects the analytes from lightâinduced degradation and leads to better sensitivities and recovery rates.

Conclusion
In summary, the presented application describes a quick and convenient method for the determination of TPM dyes from aquatic samples.
References
- BfR Expert Opinion No. 007/2008, 24 August 2007.
- Official Journal of the European Communities L 221, 17 August 2002, 8–36.
- M. Anastassiades, S.J. Lehotay, D. Stajnbaher, and F.J. Schenck, J. AOAC Int. 86, 412–431 (2003).
- SPE Application Nos. 306560, 306570 and 306580, MACHEREY-NAGEL, available from www.mn-net.com/apps.
- HPLC Application No. 128430, MACHEREY-NAGEL, available from www.mn-net.com/apps.

MACHEREY-NAGEL GmbH & Co. KG
Neumann-Neander-Str. 6 – 8, 52355 Düren, Germany
Tel.: +49 24 21 969 0 Fax: +49 24 21 969 199
E-mail:info@mn-net.com Website:www.mn-net.com


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










