Separation of Cold Medicine Ingredients Using UPLC Technology
LCGC Asia Pacific
Eric S. Grumbach, Diane M. Diehl and Jeffrey R. Mazzeo, Waters Corporation
Pharmaceutical formulations used to treat the common cold often contain multiple active ingredients to treat different symptoms. These actives can include combinations of decongestants, antihistamines, pain relievers, cough suppressants and expectorants in addition to numerous excipients, all of which exhibit different chemical properties, including polarity. It is this wide range of analyte polarities that often makes chromatographic methods development difficult.
UPLC technology was used to develop a single chromatographic method for the analysis of 20 of the most common pharmaceutical formulations targeted to relieve symptoms associated with the common cold. A new high strength silica (HSS) UPLC stationary phase was used to develop a single chromatographic method for the analysis of a number of possible formulation compositions. This stationary phase was selected because of its ability to enhance the retention of polar analytes while also having good chromatographic selectivity of hydrophobic species.
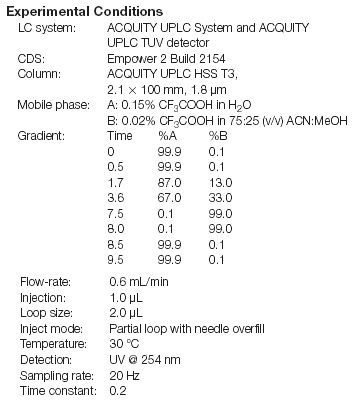
Sample Preparation
Reference standards were prepared in a solution of 75:25 (v/v) water:methanol containing 0.2% formic acid at a concentration of 25 μg/mL for each component, except acetaminophen, which was 12.5 μg/mL and its respective impurities, 4-aminophenol, 4-nitrophenol and 4-chloroacetanilide, were prepared at a concentration of 2.5 μg/mL

Figure 1
Results and Discussion
A mixture of standards of 20 common components of cold medicine formulations including active ingredients, impurities and counter ions, was separated on a 2.1 × 100 mm, ACQUITY UPLC HSS T3, 1.8 μm column, as depicted in Figure 1. A listing of elution order, relative retention, and USP resolution of all components is listed in Table 1. Retention factors range from 0.52 to 10.47. All components were baseline separated in less than 7 minutes and had resolution factors of 1.65 or greater.

Table 1
Conclusions
A fast, high resolution chromatographic method was developed for pharmaceutical formulations targeted to relieve symptoms of the common cold by utilizing a new high strength silica (HSS) UPLC stationary phase. This single chromatographic method can be used to rapidly analyse a number of possible formulation compositions containing different active ingredients.
© 2007 Waters Corporation. Waters, UPLC, ACQUITY UPLC, Empower, and The Science of What's Possible are trademarks of Waters Corporation.
Eric S. Grumbach, Diane M. Diehl and Jeffrey R. Mazzeo, Waters Corporation, Milford, Massachusetts, USA.

Waters Corporation
34 Maple Street, Milford, Massachusetts 01757, USA
tel. +1 508 478 2000 fax +1 508 478 1990
Website: www.waters.com

Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.
Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














