Sample Preparation for Food Contaminant Analysis
Examples of a wide variety of sample preparation techniques for the analysis of trace-level contaminants in food and the advantages and disadvantages of each are discussed in this article.
Food samples cover a wide range of physical matrix types and the range of potential chemical contaminants are equally diverse. Some contaminants, such as banned substances, require analysis at extremely low levels to 'demonstrate' that they are absent and the method of analysis must enable determination at trace and ultra-trace levels. The method of analysis is usually chromatographic and to have any practical validity, good sample extraction and preparation are vitally important to enable separation of the contaminant from the complex matrix and reduce the potential impact of any matrix effects. A variety of sample extraction techniques are available to the analyst and the method of choice will depend on the matrix type and analyte(s) of interest. The move towards microextraction techniques or analysis with little or no sample preparation aims to provide a more "environmentally friendly" approach by using less solvents, but must be used with caution for a complex, naturally non-homogeneous matrix, such as food. Examples of sample preparation techniques used for analysis of trace-level contaminants in foodstuffs are outlined and the advantages and disadvantages of each are assessed.
Food contaminants cover a diverse range of compounds from a number of potential origins. They can be present at trace and ultra-trace levels and therefore identification and quantification in foods can present a challenge to the analytical chemist. Many known contaminants are at levels of toxicological concern, or a maximum level permitted by legislation, but for banned substances the analytical method must be capable of measuring as low as is reasonably practicable to demonstrate absence. Moreover, for unknown contaminants, such as those responsible for taints and off-flavours, the method must also provide unequivocal identification of the causative compound(s).

As a result of the variety of analytes that can be regarded as 'food contaminants', including pesticides, packaging contaminants, environmental contaminants, processing contaminants, mycotoxins and veterinary drugs, several methods of extraction are generally required. The choice of sample preparation techniques used will depend on the contaminant(s) of interest, the food matrix and the final analytical determination/instrumentation to be used (1). The requirements of the analysis must always be considered and the method used should be fit for purpose — in terms of identification of unknown analytes, accuracy required (qualitative or quantitative) and sensitivity if working to known limits.
Typical steps in an analytical method include sampling/homogenization, extraction, clean-up, and concentration before the final analysis. A simplified schematic showing the options for both solid and liquid samples is shown in Figure 1.
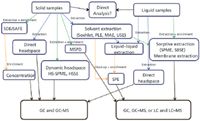
Figure 1: A simplified schematic showing the sample preparation options for both solid and liquid samples.
For the majority of food contaminants, the final analysis is performed using a chromatographic technique, combined with an appropriate detector. The increased use of mass spectrometric detectors has enabled selectivity and sensitivity to be optimized at the detection stage and has led to the development of methods with minimal sample preparation. Such approaches provide rapid methods and can cover a wide range of analytes, with crude extractions. It should be noted, however, that good sample extraction and preparation are still important, and a 'cleaner' extract will improve chromatography, reduce instrument maintenance and, in particular for liquid chromatography mass spectrometry (LC–MS), reduce the potential impact of matrix effects.
Matrix Considerations
Food can encompass a number of matrix types, and may include different biological matrices, such as meat, fish, grain, seeds and plant material, as well as dry powders, fats and liquids. The complexity of food products means that there is the potential for many interferences with the analytes of interest and that different techniques may be required depending on the matrix. When looking for trace-level contaminants, large concentrations of matrix components can cause problems, such as ethanol for alcoholic drinks.
Foodstuffs are naturally non-homogenous and this presents a challenge given that a lot of modern techniques have been developed with smaller sample sizes in mind. It is essential to ensure that any sample taken for analysis is representative of the bulk matrix. A large sample size may be needed to obtain the necessary sensitivity, although in some cases, such as a complaint from a customer, this may not be possible. When analysing for trace and ultra-trace levels of known contaminants, increased method selectivity (that reduces potential matrix interferences) can help provide the sensitivity required to determine the analytes of interest. However, for the determination of unknown contaminants, analysis becomes more of a challenge as selective extraction may not be possible. In this instance, a more investigative approach is required.
Headspace Analysis
Where an analyte is known (or suspected to be) volatile then headspace analysis can be used. This technique has the advantage of being suitable for all matrix types and uses little or no solvents. The headspace of a sample is injected directly into a gas chromatograph connected to a suitable detector and it is therefore a clean technique and avoids the non-volatile components entering the chromatographic system. Direct static headspace involves the incubation of a sample (usually with heating and shaking) for a defined period of time (ideally until equilibrium is reached). The amount of analyte in the headspace is directly proportional to the amount in the sample. This technique suits volatile and semi-volatile analytes because temperatures and incubation times can be adjusted accordingly and salt can also be added to encourage analytes into the vapour phase. However, as only a proportion of the vapour phase (headspace) is injected, direct headspace provides only limited sensitivity. A summary of headspace GC analysis for the determination of food contaminants was published in 1984 (2) and it has been reported more recently to determine 1,3-dichloropropanol in soy sauce (3) and furan in food stuffs (4).
The distribution of an analyte between the sample and gaseous phase will vary with the matrix and for quantitative work, matrix-matched standards or the use of the method of standard additions is recommended. An internal standard (IS) of similar volatility, or preferably isotopically labelled, can also be used. There are several approaches to increasing the sensitivity in headspace extraction. One approach is to use multiple headspace extraction (MHSE) and perform sequential headspace analysis from the same vial. This can provide exhaustive extraction of an analyte and theoretical amounts can be calculated from a limited number of consecutive extractions.
Another approach to enable a more exhaustive extraction of the sample is to use dynamic headspace or gas sampling techniques. This enables enrichment of volatile analytes in a cold trap or on an inert support by continually flushing the headspace of the sample. Thermal desorption enables direct transfer of the analytes into the GC instrument following extraction.
Traditional dynamic headspace (purge and trap) GC is rarely used for trace contaminant analysis in foods. This is largely a result of the lack of selectivity that it offers as, although large concentration factors are possible, any interferences present are also concentrated and therefore increases in sensitivity can be limited. However, the use of more selective absorbent materials as traps may help overcome this and additional cryo-focusing steps help to minimize losses before injection. Because of the flows needed on some systems to desorb the trap, a split is required, leading to additional potential losses in sensitivity. Applications using commercially available needle-trap systems are increasing, as illustrated in a recent review of needle trap and related techniques (5). It has been reported for analysis of food volatiles (6), although food applications are limited and, as with all miniaturized techniques, limitations in sample size have to be considered to ensure that any sample is representative.
Sorptive Extraction
Sorptive extraction techniques are based on the extraction of analytes from the matrix into a non-miscible extracting phase, which can be coated on a solid support. Sorption can include extraction onto active sites on the surface (adsorption) or partition of the analytes into the extracting phase (absorption), or a combination of both. The main advantages of sorptive extraction compared to some other extraction techniques are the reduction in solvent use, the combination of sampling and extraction into one step and the high enrichment factors that can be achieved.
As with headspace analysis, sorptive extraction techniques are generally equilibrium based and do not provide an exhaustive extraction. However, although the maximum sensitivity is achieved at equilibrium, extractions can be performed for a defined period of time, providing conditions are kept consistent. As with headspace, the use of ISs, matrix-matched standards or the method of standard additions is recommended for accurate quantitation. Some techniques enable pre-doping of the extraction phase with standards and, in some cases, kinetic calibration regimes have been reported to improve quantitation (7).
Solid-Phase Microextraction
Solid-phase microextraction (SPME) is a commonly used sorptive extraction technique. It can be used to sample liquids or solutions directly, or to analyse the headspace above a sample. For food contaminant analysis direct immersion SPME is rarely used because of issues with fouling of the fibre or interference from a high concentration of matrix components. Headspace SPME sampling enables analysis of almost any matrix for volatile and semi-volatile compounds. The fibres are available with different extraction coatings and the process can be automated using a modified microsyringe. Following extraction, the fibre is desorbed thermally directly into a GC, or can be eluted with solvent for subsequent GC or LC analysis.
Fibre coatings can be pure liquid polymers [such as polydimethylsiloxane (PDMS) or polyacrylate (PA)] or mixed films containing liquid polymers and solid particles [such as carboxen-PDMS]. The choice of phase will depend on the properties of the analyte (if known). A mixed fibre, such as divinylbenzene(DVB)/carboxen-PDMS, enables extraction of a wide range of analytes and is often used for volatile screening methods. PDMS is also a commonly used phase because it has generic selectivity for many types of non-polar analytes, but for more polar analytes, polyacrylate and carbowax-divinylbenzene (CW-DVB) are more suitable. Alternative coating materials are also available that provide extraction for specific analytes, including affinity coatings and chiral coatings for optically active analytes. It should be noted that when using headspace SPME, because of the partitions involved (as illustrated in Figure 2), more volatile compounds may be lost from the fibre because they will favour the vapour phase at increased temperature. This can be a useful tool for selective analysis, as the fibre will favour lower volatility compounds than direct headspace. Cold fibre SPME, (CF–SPME), enables the fibre to be cooled while the sample is heated, and is thus reported to increase extraction efficiencies. Derivatization can be used to maximize extraction efficiencies for some analytes, either prior to extraction or on-fibre.
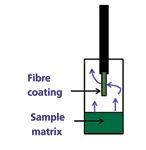
Figure 2: Partitions in HS-SPME between sample matrix, vapour and fibre.
SPME offers an increased sensitivity compared with direct headspace because of the high enrichment factors that can be achieved. Moreover, as a result of the different selectivity, HS–SPME is most commonly used to obtain volatile/semi-volatile profiles, particularly in flavour analysis. Applications for the determination of trace contaminants in foods include determination of furan in baby food (8), phthalates in wine (9) and ham sausage (10) and PAHs in tea (11).
However, one of the drawbacks of the technique can be the limited volume of stationary phase that can be bound to the fibre.
Stir Bar Sorptive Extraction
To overcome the limited extraction capacity of SPME fibres, stir bar sorptive extraction was developed. This technique uses a glass stir bar, which can be coated with a larger volume of stationary/extracting phase than a SPME fibre. The 'Twister' bars are coated with a bonded adsorbent layer of PDMS and, following extraction, can be thermally desorbed directly into a GC or eluted with solvent for subsequent analysis by GC or LC. Unlike SPME fibres, twisters are more often used for direct sampling, but can also be used for headspace sampling to provide additional selectivity and enrichment. Because of the non-polar nature of the PDMS, this extraction technique works best for analytes with octanol-water distribution coefficients between 100,000 and 100 (log Kow between 2 and 5). As the technique can be used for liquid or semi-solid samples, it is suitable for many food types and can also be used following an initial extraction. To enable extraction of the more polar analytes, the matrix can be modified or derivatization can be used. Currently, alternative coatings are being developed to offer different selectivity. Long extraction times may be required for exhaustive extraction and generally this technique is considered an equilibrium-based extraction and therefore optimization of conditions is required for accurate quantitation. The use of matrix matched standards or ISs are recommended. For food contaminant analysis, to date, applications are mainly for non-fatty food matrices and non-polar analytes, although some multi-analyte methods have been reported (12, 13).
Sample Preparation: Solid Samples
For solid samples, the efficiency of extraction depends on penetration of the sample by the solvent, therefore an initial homogenization step, such as drying and grinding, is often used. Following this there are a number of extraction techniques available.
Matrix Solid-Phase Dispersion
One technique which enables homogenization and extraction in one step for solid or liquid samples is matrix solid-phase dispersion (MSPD). With this approach, the sample is mixed with a sorbent, such as C18-bonded silica or hydromatrix, and the analytes eluted with solvent. The porous structure provided by the matrix aids penetration by the extraction solvent and can also have some functionality which can retain unwanted matrix components, such as fats/lipids. This technique is particularly useful for samples with a high proportion of fats, such as animal tissue samples, where drying can be problematic and is commonly used in pesticide analysis (14–18). A review of MSPD for extraction of contaminants from foodstuffs was published in 2007 (19).
A number of techniques that have been used for extraction of contaminants from solid samples are outlined below.
Solvent Extraction Techniques For Solid Samples
Soxhlet extraction has been widely used for the extraction of thermally stable analytes from foodstuffs, such as polyaromatic hydrocarbons (PAHS) and polychlorinated biphenyls (PCBS) (20,21). It provides an exhaustive extraction by the continuous cycling of solvent through the heated sample matrix, with collection of analytes into the hot solvent. Automated systems enable several simultaneous extractions to be performed. To improve extraction efficiencies, soxhlet can be combined with microwave or ultrasonic extraction. A related technique: pressurized liquid extraction (PLE) – uses elevated pressures, which enables a more rapid extraction because the solvent remains in the condensed state. Alternative names of PLE include accelerated solvent extraction (ASE), high-pressure solvent extraction (HPSE), pressurized hot solvent extraction (PHSE) and subcritical solvent extraction (SSE).
If water is used as an extraction solvent it can be known as super-heated water extraction (SHWE), subcritical water extraction (SWE), hot water extraction (HWE), pressurized hot water extraction (PHWE) or high temperature water extraction (HTWE). As a result of the changes in polarity of water at high temperature, this can have additional advantages. The use of a supercritical fluid (SFE) (such as carbon dioxide) as an extraction solvent provides another green alternative and enables the easy removal of the 'solvent' following extraction.
The selectivity of these solvent extraction techniques can be improved by the addition of sorbents to the extraction cells or by modifying the extraction solvent. Microwaves and ultrasonic extraction can be employed to enhance extraction efficiencies. For microwave-assisted extraction (MAE), at least some polar solvent needs to be used, and as the sample is heated it is only suitable for thermally stable analytes. Most of these extraction techniques are not suitable for volatile analytes. A wider choice of extraction solvent can be used for ultrasonic solvent extraction (USE) and the technique needs no specialized equipment so is relatively inexpensive. However, although multiple extractions can be performed simultaneously, it is not easily automated.
Using these techniques for the analysis of food contaminants, means that further steps following extraction are invariably required to enable analyte enrichment and/or concentration. Reported applications for food contaminant analysis include PLE for determination of Bisphenol A diglycidyl ether (22), ochratoxin A (23) and various persistent organic pollutants (24), SHWE for selected pesticides from fruit and vegetables (25), SFE for pesticides (26,27) and PAHs (28). The use of USE for pesticides (29,30), glycyrrhizic acid from liquorice root (31) and quinolones in pig muscle has been reported (32). Determination of contaminants in food and soil has been reviewed by Tadeo et al (33).
Complete extraction solutions are becoming more common and enable on-line cleanup; for example, PLE systems linked to a solid phase extraction (SPE) cartridge or disk to allow for selective clean-up and enrichment directly following extraction.
For solid samples, following the extraction step, the extracts can then be treated as liquid samples and further extraction/cleanup procedures can be used prior to analysis.
Extraction Techniques: Liquid Samples or Solid Sample Extracts
For liquid samples, or solutions obtained following an initial extraction, analytes can be partitioned directly into an immiscible extracting phase. For non-homogeneous samples this can be preceded by a dissolution or homogenization step and possibly filtration.
Direct partitioning liquid-liquid extraction (LLE) enables an analyte to be selected based on its solubility or affinity for the immiscible extracting phase compared to the sample matrix. Traditional methods use a separating funnel, and after mixing the phases are allowed to separate and the phase containing the analyte is retained (and invariably concentrated). The selectivity of the technique is through choice of extraction solvent. To improve efficiency of extraction, matrix modification such as addition of salt or change in pH can be used and, if necessary, centrifugation to ensure a distinct phase barrier is achieved.
As the extraction solvent can be chosen to suit the analyte properties, direct liquid extraction can be used for the majority of food contaminants, although with the complexity of some food matrices, the challenge can be the preferential extraction of analytes versus unwanted matrix components. For trace contaminants in foods a further concentration step and possibly cleanup (such as solid phase extraction) is generally required and this is one of the major disadvantages of traditional liquid-liquid extraction. Where a food contaminant is unknown, for example, in the case of taint analysis or investigations into customer complaints or suspected adulteration, isolation from food matrix components can be a challenge. For this analysis and also for multi-class or multi-residue methods, a compromise in the choice of solvent is generally made to give optimum extraction for the most number of compounds.
To enable analytes to be exhaustively extracted from the samples, multiple extractions are often required and therefore large volumes of solvents may be needed. As a result miniaturized liquid extraction techniques have now been developed.
Liquid-Phase Microextraction (LPME)
Liquid-phase microextraction (LPME) involves the partition of analytes between the bulk aqueous phase and a very small volume of extraction solvent. In single-drop microextraction (SDME), a single drop of organic solvent is suspended at the tip of a needle in the sample solution. This extraction can be performed in static or dynamic mode, or the drop can be protected by a hollow fibre. This technique can also be used for headspace analysis to enhance selectivity for volatile analytes (34). The developments and applications of SDME were the subject of a review in 2010 (35).
The choice of extraction solvent is limited because it has to have sufficient viscosity to avoid it dropping off the needle, and in headspace mode the solvent must have a boiling point high enough to avoid evaporation. The use of an IS is recommended for accurate quantitation. There are only a few reported methods that have been developed for food contaminant analysis, including ochratoxin in wine (36), herbicides in milk (37) and organophosphorus pesticides in orange juice (38). A related approach known as dispersive liquid-liquid microextraction (DLLME), mainly used for analysis of water, uses a mixture of two organic solvents (extractant and dispersant).
This technique has recently been reported for multiresidue determination in fruit juices (39) and fungicides in wine (40). It has also been combined with USE for extraction of pesticides from tomatoes (41).
Continuous Liquid Extraction/Distillation (SDE and SAFE)
Distillation can also be considered an extraction because it separates components based on boiling points and vapour pressures. Steam distillation extraction is a technique that provides a continuous extraction and involves the co-distillation of the sample between two solvents. The sample (usually diluted with water) is heated in one flask while the extraction solvent is boiled in another. The vapours mix in a central chamber and the volatile compounds are extracted into the solvent over time. Various modifications of the original apparatus developed by Likens and Nickerson have been made, including microscale and vacuum SDE apparatus. A related technique is solvent-assisted flavour extraction or evaporation (SAFE). This uses a vacuum pump to enable extraction at lower temperatures and helps to avoid artefact formation or loss of thermally unstable analytes. SDE and SAFE extract the volatile components of a sample, without the non-volatile food matrix components and are often used in flavour and taint analysis (42, 43). Published applications include volatiles in cocoa powder (44), chloroansioles in raisins (45) and 2,6-dibromophenol in crustacean (46). High enrichment factors can be achieved because large sample sizes can be used, although for trace contaminants a further concentration step is often still required. The major disadvantage of these approaches is the time taken for analysis and the need for specialist glassware, meaning only a few samples can be extracted simultaneously. Although widely used for flavour characterization and taint analysis, SDE or SAFE are not routinely used for determination of known contaminants in food.
Membrane Extraction (ME)
To increase the selectivity of LLE for known contaminants, membrane extraction (ME) can be used. In this technique, a membrane is placed between two immiscible phases and acts as a porous or non-porous barrier, enabling extraction into a small amount of solvent (receiving or extracting phase). Separation can be based on size for porous membranes or size and charge if ion exchange (IEX) membranes are used. Non-porous membranes contain a liquid, which the analytes actually dissolve in to, providing a true 'extraction' based on the partition of the analytes between the phases. Hollow fibre membranes enable the support of an organic extracting solvent during extraction and various systems, including three-phase and dynamic modes, have been reported. The equilibria between phases can be controlled to extract the analyte of choice, by the use of buffers, complexing agents or derivatization.
Advances and developments in membrane extraction for GC have been reviewed (47) and high enrichment factors can be achieved by using a small volume of extracting solvent (although longer extraction times may be required). Current applications of membrane extraction for analysis of contaminants in foods are limited mainly to beverages, and the technique has been reported for extraction of PAHs (48) and fungicides in wine and juices (49). More robust automated systems are now available, potentially leading to an increase in food analysis applications. The technique has also been reported in combination with molecularly imprinted solid-phase extraction (50).
Cleanup/Enrichment: Solid-Phase Extraction (SPE)
Solid-phase extraction (SPE) can be used as both a clean-up and enrichment technique and generally follows a solvent extraction step. Analytes are extracted by partitioning between a solid sorbent surface and the sample matrix (liquid phase). It is designed to provide exhaustive extraction, although the process must be reversible to enable subsequent elution of the target analytes.
Glass columns packed with sorbent can be used, but polymeric SPE cartridges are more commonly employed. A large variety of apparatus is available to suit different applications, including cartridges, discs and disposable plastic pipette tips fitted with sorbent beds. The wide range of sorbents available mean that there are many possibilities for targeted analysis and selectivity can be changed by adjusting the pH, solvent composition and the surface chemistry of the sorbent material. Extraction can be based on adsorption, polar and non-polar interactions, cation or anion exchange or size exclusion. Mixed mode sorbents are also available and for targeted analysis very specific sorbents have been developed: for example, based on antibodies (immunosorbents) or molecularly imprinted polymers (MIPS).
The choice of SPE cartridge will depend on the analyte properties and the matrix and can have a significant affect on recoveries and also the ultimate sensitivity of the analytical method. The sample matrix can affect the efficiency of the sorbent to extract the analytes, particularly where there is competition for retention. Efficient cleanup procedures are particularly important for methods where the final analysis is achieved by LC–MS and matrix interferences can affect ionization efficiency.
Although there are many SPE cartridges available covering most classes of analytes, for extraction of some food contaminants, a multistep approach is required, utilizing more than one cartridge to provide optimum selectivity and enrichment. Highly selective sorbents such as MIPS, which were developed to mimic the specificity of immunosorbents and retain the analytes based in specific cavity as a result of shape recognition, tend to be more stable to heating, pH ranges and organic solvents and have also been used in food contaminant analysis (51). Published examples include multi-class vet drugs in shrimps (52) and the use of immuno sorbents for selected mycotoxins (53).
Automated SPE systems are now widely available, although for some matrices reproducibility can still be an issue. The use of the optimum SPE cartridge for targeted analysis can be key to method performance and not only improve sensitivity of the method by reducing background and increasing selectivity, but also improve robustness and minimize instrument maintenance required.
For unknown contaminants generic sorbents can be used or, if time permits, then using on-line prep systems, a multi cleanup approach can be used –trying several cartridges and comparing results. A multi-step approach using more than one type of sorbent may be required for some matrices and contaminants.
However, for one-off analysis, or where sample size is limited, this is often difficult as a result of time constrictions. Therefore, as in many cases in investigative analysis, a compromise is made. It may be that a more robust approach is developed following initial identification of the analyte, to enable more rapid follow-up analysis or continued monitoring.
Generic Protocols (QuEChERS)
Initially developed for pesticide analysis, a generic protocol such as QuEChERS (Quick Easy Cheap Effective Rugged Safe) (54) which uses a MSPD approach, has been successfully used for multi-analyte/multiclass methods. The resultant extracts are suitable for GC and LC–MS analysis and various modified versions of the basic method have been presented for a range of known food contaminants including pesticides (55). Such approaches that use minimal sample preparation are on the increase, but without any cleanup for some food stuffs, this 'dilute and shoot' approach can result in poor chromatography and increased instrument maintenance. For known contaminants, however, where method performance can be assessed, generic protocols may provide an initial step in development of an optimized analytical method.
Direct Analysis
Developments in direct ambient ionization mass spectrometry techniques have led to methods with no sample preparation or chromatography at all, and applications in food analysis are increasing. These include direct analysis in real time (DART), desorption electrospray ionization (DESI) and atmospheric solids analysis probe (ASAP). These recent innovations have the ability to record mass spectra on samples without any sample preparation. Current applications for contaminants in food are limited, although such approaches have been reported for determination of melamine in milk (56) and oil authenticity (57). DESI–MS is being used increasingly as a screening tool and its use for analysis of contaminants in foods was recently reviewed (58), although it was noted that some sample preparation is likely to be required for most applications. These techniques can also be used with SPME or SBSE sampling as the fibre or stir bar can be held directly in front of source.
Direct techniques such as these are useful for developing rapid screening approaches, although for complex matrices — particularly food, which is naturally inhomogenous, as only a portion of the sample is exposed to the source —reproducibility and accurate quantitation can be a challenge. When used to monitor specific ions (e.g. for explosive or drug applications) a rapid result can be achieved, but for trace and potentially unknown contaminants in food the current approaches are less suitable.
Conclusions
The choice of any analytical method should always be fit for purpose (be that identification, qualitative comparison or accurate quantitation for measurement to a specific regulatory limit). For known contaminants with a defined limit, very targeted approaches can be developed. For multi-residue and multi-class methods, a compromise is often required for optimum performance for the largest number of compounds. In some cases it is worth spending the time on sample preparation to enhance method performance, rather than use a 'quick and dirty' approach and have problems with matrix effects and instrument maintenance. Developments in on-line sample preparation enable rapid, robust methods to be developed that are suitable for high-throughput procedures. However, where selective extraction is not an option (as the target analytes are unknown), developments in instrumentation mean that some selectivity can be achieved at the detection stages during final analysis.
Kathy Ridgway is a technical specialist in the investigative analysis department of Reading Scientific Services Ltd. A graduate of the University of Surrey, she has over 16 years laboratory experience and has worked on the determination of a variety of chemical contaminants in foods. Prior to joining RSSL she worked for Unilever for over 10 years, were she completed a PhD at the University of Loughborough. She now works as a technical specialist at RSSLspecializing in the determination of compounds responsible for food taints and off-flavours and in understanding the origin of such compounds.
References
(1) K. Ridgway and S.P.D. Lalljie, R.M. Smith, J. Chromatogr. A, 1153(1–2), 36–53 (2007).
(2) J. Gilbert, ed., Analysis of food contaminants, Elsevier Applied Science, Essex, 1984, 117 (1984).
(3) C. Crews, G. Lebrun and P.A. Brereton, Food Addit. Contam., 19(1), 343–50 (2002).
(4) A. Becalski, D. Forsyth, V. Casey, B.P.Y. Lau, K. Pepper and S. Seaman, Food Addit. Contam., 22(6), 535–40 (2005).
(5) L.H. Lord, Z. Weigiang and J. Pawliszyn, Anal. Chim. Acta, 677 (1), 3–18 (2010).
(6) C. Bicchi et al., J. Chromatogr. A, 1184(1–2), 220–233 (2008).
(7) G. Ouyang and J. Pawliszyn, Anal. Chim. Acta., 627(2), 184–97 (2008).
(8) F. Bianchi, M. Careri, A. Mangia and M. Musci, J. Chromatogr. A, 1102(1–2), 268–72 (2006).
(9) M. Del Carlo et al., Food Chem., 111(3), 771–777 (2008)
(10) Z. Guo et al., Meat Sci., 84(3), 484–490 (2010).
(11) P. Viñas et al., J. Chromatogr. A, 1164(1–2), 10–17 (2007).
(12) K. Ridgway, S.P.D. Lalljie and R.M. Smith, Anal. Chim. Acta., 657(2), 169–174 (2010).
(13) L. Maggi et al., J. Chromatogr. A, 1209(1–2), 55–60 (2008).
(14) A.I. Valenzuela, R. Lorenzini, M.J. Redondo and G. Font, J. Chromatogr. A, 839(1–2), 101 (1999).
(15) Q.H. Zou, Y. Liu, M.X. Xie and J. Han, L. Zhang, Anal. Chim. Acta., 551(1–2), 184 (2005).
(16) V.I. Valsamaki, V.I. Boti, V.A. Sakkas, and T.A. Albanis, Anal. Chim. Acta., 195, 573 (2006).
(17) C. Ferrer, M.J. Gomez, J.F. Garcia-Reyes, I. Ferrer, E.M. Thurman and A.R. Fernandez-Alba, J. Chromatogr. A, 1069(2), 183 (2005).
(18) C. Blasco, G. Font and Y. Pico, J. Chromatogr. A, 970(1–2), 201, (2002).
(19) S. Bogialli and A. Di Corcia, J. Biochem. Biophys. Methods, 70(2), 163–179 (2007).
(20) K.E.C. Smith, G.L. Northcott and K.C. Jones, J. Chromatogr. A, 1116(1–2), 20–30 (2006).
(21) M.O. Punin Crespo, M.A and Lage Yusty, Chemospher., 59(10), 1407–13 (2005).
(22) O. Pardo, V. Yusa, N. Leon and A. Pastor, J. Chromatogr. A, 1107(1–2), 70–78 (2006).
(23) L.G. Osnaya, J.M.S.d. Castillo, J.C.M. Cortes and J.M. Vinuesa, J. Chromatogr. A, 1113(1–2), 32–36 (2006).
(24) E. Bjorklund, S. Sporring, K. Wiberg, P. Haglund and C.V. Holst, TrAC Trends Anal. Chem., 25(4), 318–25 (2006).
(25) R.M. Smith, J. Chromatogr. A, 975(1), 31–46 (2002).
(26) V.G. Zuin, J.H. Yariwake and C. Bicchi, J. Chromatogr. A, 985 (1–2), 159–66 (2003).
(27) S.R. Rissato, M.S. Galhiane, F.R.N. Knoll and B.M. Apon, J. Chromatogr. A, 1048(2), 153–59 (2004).
(28) M.A. Lage Yusty and J.L. Cortizo Davina, Food Control., 16(1), 59–64 (2005).
(29) M. Barriada-Pereira, M.J. Gonzalez-Castro, S. Muniategui-Lorenzo, P. Lopez-Mahia, D. Prada-Rodriguez and E. Fernandez-Fernandez, Talanta, 71(3), 1345–51 (2007).
(30) I. Rezic, A.J.M. Horvat, S. Babic and M. Kastelan-Macan, Ultrason. Sonochem., 12(6), 477–81 (2005).
(31) X. Pan, H. Liu, G. Jia and Y.Y. Shu, Biochem. Engineer. J., 5(3), 173–77 (2000).
(32) M.P. Hermo, D. Barron and J. Barbosa, Anal. Chim. Acta., 539 (1–2), 77–82 (2005).
(33) J.L. Tadeo, C. Sánchez-Brunete, B. Albero and A.I. García-Valcárcel, J. Chromatogr. A, 1217(16), 2415–40 (2010).
(34) D.C. Wood, J.M. Miller and I. Christ, LC-GC Europe, 17, 573–579 (2004).
(35) M.A. Jeannot, A. Przyjazny and L. Hou, G. Shen and H.K. Lee, J. Chromatogr. A, 985(1–2), 107 (2003).
(36) L. Hou, G. Shen and H.K. Lee, J.Chromatogr A, 985(1–2)107 (2003).
(37) A. Bjorhovde, T.G. Halvorsen K.E. Rasmussen and S. Pedersen-Bjergaard, Anal. Chim. Acta., 491(2), 155 (2003).
(38) E. Zhao, L. Han, S. Jiang, Q. Wang and Z. Zhou, J. Chromatogr. A, 1114(2), 269–73 (2006).
(39) M. Guzalnur et al., J. Chromatogr. B, 879(22), 2113–2118 (2011).
(40) T. Rodriguez-cabo, I. Rodriguez, M. Ramil and R. Cela, J. Chromatogr. A, (in press) (2011).
(41) A. Bidari et al., Food Chem., 126(4), 1840 (2011).
(42) H. Maarse, Food Taints and Off-flavours, M.J. Saxby, ed., Blackie academic & professional (Chapman and Hall): Chapter 3 (1993).
(43) K. Ridgway, S.P.D. Lalljie and R.M. Smith, Food Addit. Contam., 27(2), 146–68 (2010).
(44) D.J. Caven-Quantrill and A.J. Buglass, J. Chromatogr. A, 1117(2), 121–31 (2006).
(45) C. Bicchi, C. Iori, P. Rubiolo and P. Sandra, J. Agric. Food Chem., 50(3), 449–59 (2002).
(46) V.G. Zuin, M. Schellin, L. Montero, J.H. Yariwake, F. Augusto and P. Popp, J. Chromatogr. A, 1114(2), 180–87 (2006).
(47) T. Thaer Barri, and J-A. Jan-Åke Jönsson, J. Chromatogr. A, 1186(1–2), 16–38 (2008).
(48) R. Rosario, J. Chromatogr. A, 1163(1–2), 288–297 (2007).
(49) P. Viñas et al., J. Chromatogr. A, 1194(2), 178–183 (2008).
(50) L. Chimuka, M. Van Pinxteren, J. Billing, E. Yilmaz and J.Å.Jönsson, J. Chromatogr. A, 1218(5), 647–653 (2011).
(51) C. Baggiani, L. Anfossi and C. Giovannoli, Anal. Chim. Acta., 591(1), 29–39 (2007).
(52) C. Molins-Legua and F. Campins, Anal. Chim. Acta., 546(2), 206 (2005).
(53) E. Watanabe, Y. Yoshimura, Y. Yuasa and H. Nakazawa, Anal. Chim. Acta., 433(2), 199 (2001).
(54) M. Anastassiades, S.J. Lehotay, D. Stajnbaher and F.J. Schenck, J. AOAC Int., 86(2), 412–31 (2003).
(55) A. Wilkowska and M. Biziuk, Food Chem., 125(3), 803–812 (2001).
(56) A. John Dane and Robert B. Cody, Analyst, 135(4), 696–699 (2010).
(57) J.J. Hajslova, L. Vaclavik, T. Cajka, V. Hrbek, Anal. Chim. Acta., 645 (1–2), 56–6 (2009).
(58) M.W.F. Nielen et al., TrAC Trends Anal. Chem., 30(2), 165–180 (2011).
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.









