Ruggedness of YMC Cyano-High Strength Stationary Phase Under Acidic and Basic Extremes
The Application Notebook
Silica-based cyano stationary phases have historically exhibited poor stability and short column lifetime at pH extremes. This application note details an investigation of the stability of YMC's Cyano-HG (high strength) 10 ?m silica-based stationary phase, which was performed at a customer's request.
Silica-based cyano stationary phases have historically exhibited poor stability and short column lifetime at pH extremes. This application note details an investigation of the stability of YMC's Cyano-HG (high strength) 10 μm silica-based stationary phase, which was performed at a customer's request. The columns were stressed under acidic (0.1% trifluoroacetic acid) and basic (10 mM ammonium bicarbonate, pH = 8.2) conditions, and evaluated for retention time, USP tailing, and USP plate count using two probes (naphthalene and methyl benzoate) and the following method:
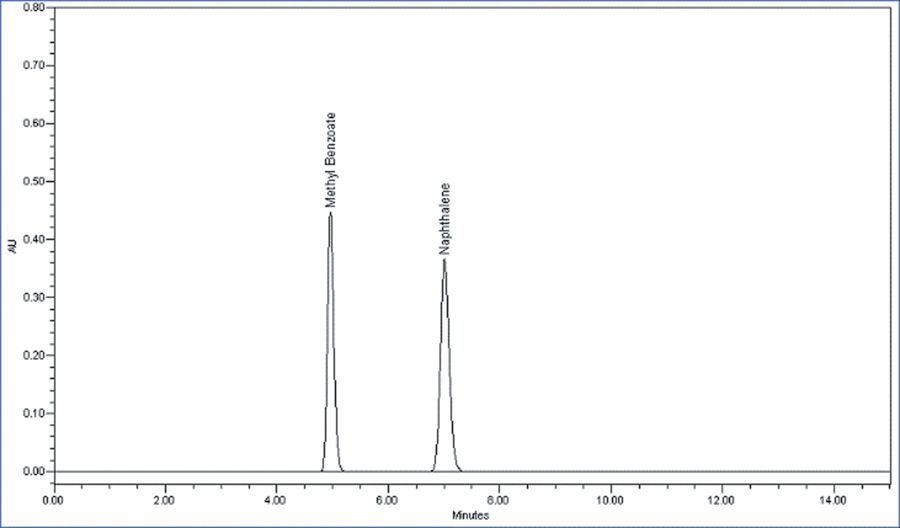
An example chromatogram for the column performance test can be seen below:
The first column was run under the basic test conditions, exhibiting a modest loss in retention time for both analytes (0.087 min for methyl benzoate and 0.265 min for naphthalene). Tailing factor remained at 1.0 for both analytes and plate count was also very stable for both over the entire 50 h span.
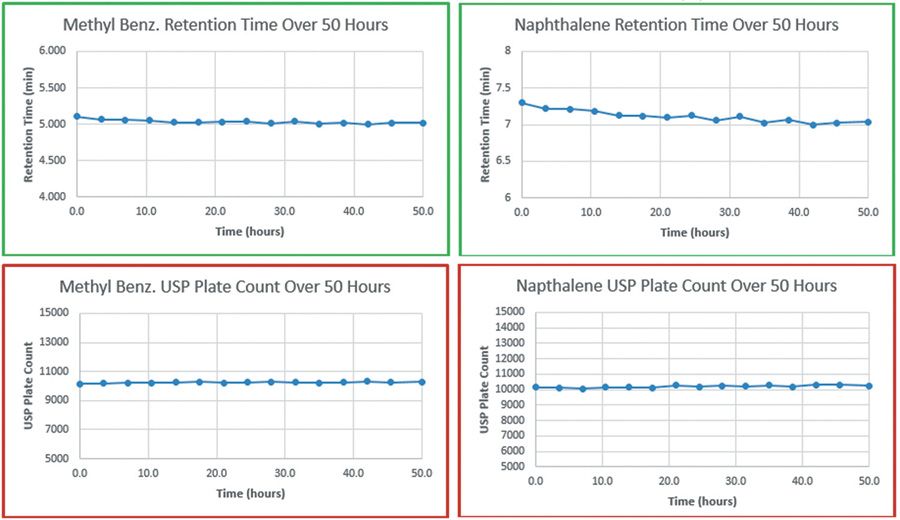
Figure 1: Cyano-HG stability - basic conditions.
The second column was tested in the same manner except under the acidic conditions. This column exhibited essentially no loss in retention time (0.010 min) for methyl benzoate and only a slight decrease in retention time for naphthalene (0.065 min). Tailing factor remained unchanged (1.2 for methyl benzoate and 1.1 for naphthalene) and plate count was very stable under these conditions, similar to what was seen during the basic stress runs.
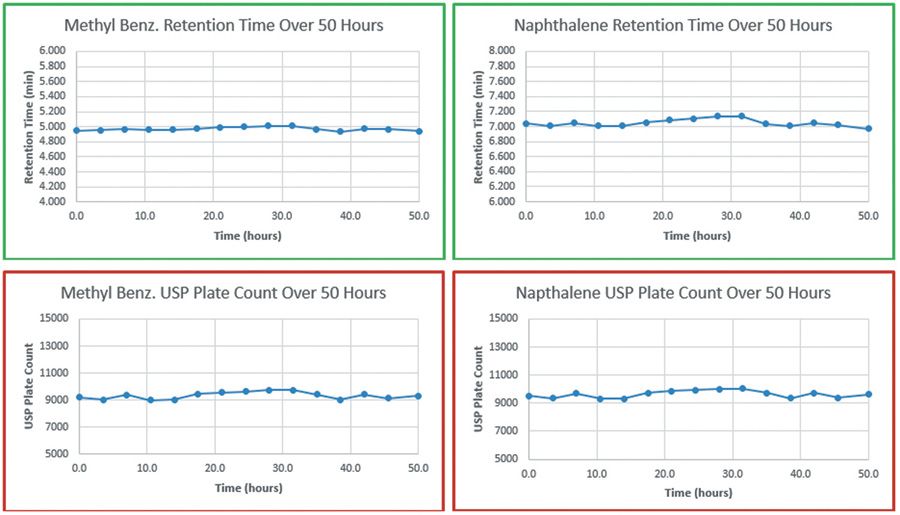
Figure 2: Cyano-HG stability - acidic conditions.
Overall, 10 μm YMC-Pack Cyano-HG stationary phase proved to be very rugged under both stress conditions and is suitable for use in preparative separations when used under the test conditions.

YMC America, Inc.
941 Marcon Blvd., Suite 201, Allentown, PA 18109
tel. (610) 266-8650
Website: www.ymcamerica.com

Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.
Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











