Fast and Robust Analysis of Drugs of Abuse Using Direct Exposure Probe on the Pegasus HT
The Application Notebook
Direct exposure probe (DEP) coupled to the Pegasus HT, time-of-flight mass spectrometer, was used for the identification of synthetic cannabinoids in MTN-series compounds.
Direct exposure probe (DEP) coupled to the Pegasus HT, time-of-flight mass spectrometer, was used for the identification of synthetic cannabinoids in MTN-series compounds.
Crime laboratories are responsible for the timely identification of seized drugs used as evidence in criminal investigations. The objective was to identify drugs of abuse using DEP instead of traditional LC or GC for time-saving and ease-of-use.
Experimental Conditions

Results
The MTN-series of compounds are allegedly blends of JWH-series compounds, but have not been studied as extensively. Very rapidly, it was determined that MTN-767 is most likely a blend of JWH-018 and JWH-081 (Figure 1). True Signal Deconvolution® produced two mass spectra that were compared to the NIST, Wiley, and Cayman library databases and yielded hits with similarity scores greater than 800 out of 1000. This result, as well as the analysis of other MTN compounds, confirmed that MTN-series compounds are blends of JWH compounds, and was accomplished within a few min.
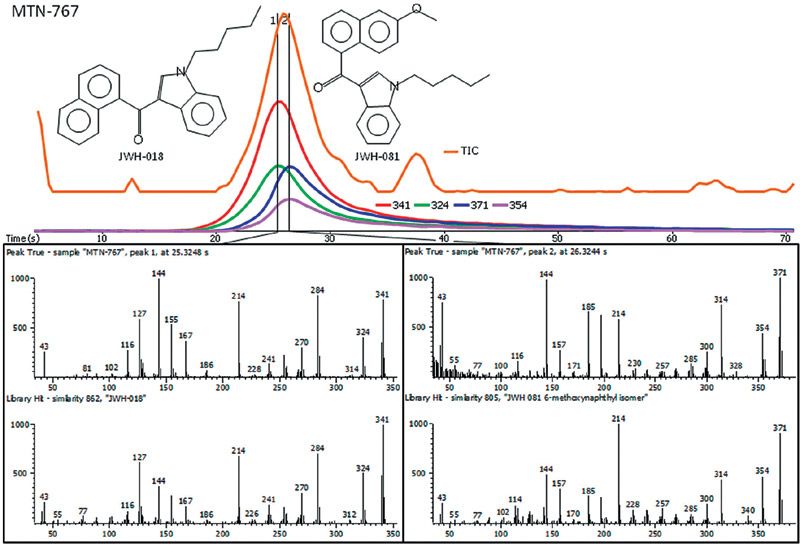
Figure 1: DEP analysis was used for the fast and reliable identification of the unknown components in MTN-767, a recreational synthetic cannabinoid.
Advantages of DEP experiments on LECO's Pegasus HT:
1. Open source design - robust and low maintenance
2. Time-of-flight MS - no mass spectral skewing
3. True Signal Deconvolution® - identify more components
4. Probe installation does not interfere with GC or GC×GC

LECO Corporation
3000 Lakeview Avenue, St. Joseph, MI 49085
tel. (269) 985-5496, fax (269) 982-8977
Website: www.leco.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











