The Role of Polymers in Solid-Phase Extraction and Sample Preparation
LCGC North America
This installment of SPP will compare and contrast the various types of polymeric and non-polymeric sorbents. The major advantages or polymeric sorbents will be discussed, and some applications will illustrate the versatility of polymeric SPE.
Solid-phase extraction (SPE) is a fast, cost-effective sample preparation technique for purifying complex samples before their analysis by gas chromatography (GC) or liquid chromatography (LC). The technique removes undesirable matrix compounds that can interfere with the analysis. In many cases, SPE has replaced liquid–liquid extraction (solvent extraction), which often uses copious amounts of organic solvent and is labor-intensive and difficult to automate, especially in its classical operation. In SPE, analytes are partitioned between a solid phase and a liquid phase and the stationary phase usually is chosen to have a greater affinity for the analytes than for the sample matrix. The classic SPE stationary phases range from bare silica and other solid sorbents such as alumina, florisil, and carbon to chemically bonded silica with phases similar to those used in high performance liquid chromatography (HPLC). Interestingly, as can be seen in Figure 1 (1,2), the usage of bonded sorbents in SPE almost mirrors the usage of the same phases in HPLC. Silica, ion-exchange phases, and affinity phases show slightly greater percentages of use in SPE. Included in Figure 1 are Florisil and alumina, non-silica-based but popular SPE packings that see applications in SPE only.

Ronald E. Majors
Bonded silicas are still the most widely used SPE packings, with several decades of applications having been developed. Although it has been debated as to who commercialized the first SPE cartridges (3), for many years before that date, researchers were using adsorbents and polymeric polystyrene–divinylbenzene (PS-DVB) ion-exchange resins to do a rough cleanup of samples before GC and LC. Only after these adsorbents were provided in convenient-to-use cartridge format did the SPE technique take off and become a popular sample preparation technique. In recent years, there has been a renewed growth in SPE, primarily driven by the introduction of newer packing materials based upon specialized polymer technology that overcomes some of the disadvantages of silica-based materials. This installment of "Sample Prep Perspectives" will explore these new polymer-based packings and compare and contrast them with popular silica-based packings. In addition, the use of polymers in other sample preparation techniques is explored. In these applications, polymers are coated on fused-silica fibers, stir bars, micropipettes, vials, and 96-well plates
Silica-Based Packings in SPE
For many years, silica-based sorbents have been the main types used in SPE. A wide variety of bonded phases (Figure 1) can be applied to just about any SPE problem encountered. However, bonded silica sorbents in SPE have some of the same disadvantages that they display in HPLC such as limited pH range (pH 2–9), and the presence of residual silanol groups. Under the appropriate pH conditions (for example, above their pKa values around 4.5–4.7), these silanol groups become ionized and are negatively charged. Positively charged compounds such as protonated amines can interact electrostatically with these ionized silanols. Such strong ionic interactions might be difficult to overcome with organic solvent elution, and analyte recovery for these polar compounds is affected.
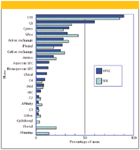
Figure 1: Comparison of phase usage in SPE and HPLC.
In the classic SPE experiment there are four main steps: conditioning the sorbent to solvate (or "wet") the stationary phase; loading the sample; rinsing away interferences; and eluting the analytes of interest. For silica gel-based sorbents and chemically bonded silica gel-based sorbents, it is fairly critical to ensure that the packing material does not dry out between steps, particularly after the conditioning step. Drying of the sorbent often "dewets" or deactivates the stationary phase and changes its properties so that analyte recovery is affected and reproducibility jeopardized. When using vacuum and positive-pressure manifolds, it is sometimes easy to allow SPE phases to dry out by inadvertently forgetting to turn off the vacuum or pressure during the critical steps.
Polymer-Based Packings in SPE
The introduction of polymeric sorbents has been a major advance in the application of SPE, especially for polar compounds. Polymers have a very wide pH range and can tolerate solvents and buffers that would harm or destroy a silica-based sorbent. In addition, they are spherical (while most silica SPE packings are shaped irregularly) and, thus, give rise to very homogeneous packed beds that display reproducible flow characteristics and minimal back pressure. The higher surface area of polymers leads to much higher sample capacity than silica. Figure 2 compares the sample capacity of LiChrolut EN, an ethylvinylbenzene-DVB copolymer SPE packing with a surface area of 1200 m2 /g, to regular silica-based SPE packings, a nonendcapped C18 (RP-18) and an endcapped C18 (RP-18e). Two solutes were investigated, caffeine and diisodecyl phthalate (DIDP). The polymeric sorbent averaged an order of magnitude increase in capacity for the more polar compound, caffeine. For the nonpolar DIDP, the increase in capacity was slightly lower. Higher capacity means that smaller sorbent bed volumes are used compared to silica packings and, thus, less sample and solvent are needed. Lower elution volumes result in higher analyte concentrations in collected fractions and better overall method sensitivity. Compared with silica-based bonded phases, polymeric phases have no such residual groups. As mentioned previously, silanol groups on the bonded surface sometimes can affect analyte retention and recovery. The absence of silanols on polymeric SPE sorbents prevents such interactions.

Figure 2: Sample capacity of LiChrolut EN in comparison with LiChrolut RP phases. The increase of sorbent capacity by a factor of at least 10 in comparison to commonly used C18 sorbent means that only 200 mg of LiChrolut EN is necessary for the complete enrichment of different contaminants from water. Courtesy of Merck KGaA, Darmstadt, Germany
Polymers are available in a variety of stationary phases with neutral PS-DVB phases, hydrophilic phases, and chemically modified phases are available readily. There are not as great a variety of polymeric phases compared to silica phases but they have become quite popular because of their advantages. The most popular are those that are neutral and whose surfaces show a good balance of hydrophobic and hydrophilic character. The hydrophilic character induced by the presence of polar functional groups, such as polyvinylpyrrolidone, polyamide, and methacrylate, contributes to the interaction with polar functional groups of analytes while the DVB portion of their backbone contributes to their high surface areas and π–π interactions with the aromatic functional groups of analytes. These "balanced" materials have been found to be useful for the simultaneous SPE of acidic, basic, and neutral compounds. Also, polymers with ion-exchange functionality such as sulfonic or tetraalkylamine allow very selective extractions of ionized or ionizable substances. Weak cation (for example, carboxyl functionality) and weak anion (for example, secondary amine) exchangers also are available. Because polymers can better tolerate the stronger buffers and wider pH values that could be used in SPE, they are preferred over silica-based ion exchangers.
Unlike silica phases, an advantage of polymeric SPE phases is the fact that they can dry out during any of the four steps and analyte recovery and reproducibility remains unaffected. Their hydrophilic surfaces wet easily with water, and sorbed analytes are unaffected because the surface never dries out or "dewets." To compare this behavior on a neutral polymeric sorbent with a polyamide-DVB character, Figure 3 compares the wet and dry results of SampliQ OPT (Optimized Polymer Technology, Agilent Technologies, Santa Clara, California) for a series of pharmaceuticals ranging from very polar basic compounds to neutral, hydrophobic compounds (4). In the "wet" experiment, the polymeric cartridge was conditioned with methanol and carried through the entire four-step process without drying out the phase. Analyte recoveries varied from 95% to 110% depending upon the compound at hand. In the second experiment, after the conditioning step with methanol, using a vacuum, air was passed through the cartridge for 10 min. All of the conditioning solvent was evaporated; upon loading the sample and performing the remaining SPE steps, analyte recovery was virtually the same when the sorbent was dried or wet. The error bars on Figure 3 shows that the relative standard deviations were quite good for these compounds, in the range of 2–3%.
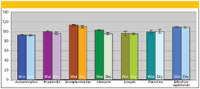
Figure 3: Recovery performance for SampliQ OPT.
Very selective analyte–sorbent interactions can occur when ion exchange is used as the predominant retention mechanism. Actually, because polymeric ion-exchange SPE phases can contain ionic functionality on a hydrophobic backbone, mixed mechanisms can occur. Such a mixed mechanism is the result of the strong ionic interaction between the positively charged groups on the sorbent (anion exchange) and the negatively charged groups on the analyte along with the hydrophobic interactions between the organic portion of the analyte of interest and the organic backbone of the polymer itself. Conversely, for a cation exchange mechanism, besides hydrophobic interactions, a positively charged analyte interacts with a negatively charged surface group. Of course, ionic interactions are much stronger than hydrophobic interactions but both types occur to some degree.
To illustrate the use of polymeric SPE sorbents to solve an application problem, the measurement of the drug amitriptyline in plasma was investigated using three different sample preparation protocols: protein precipitation, SPE on a neutral polymeric sorbent, and a cation ion-exchange polymeric sorbent (5). The structures of the sorbents are depicted in Figure 4a. In recent times, with the increased popularity of LC-mass spectrometry (LC–MS-MS) for the screening of drugs and drug metabolites in biological fluids, many pharmaceutical analysts have turned to protein precipitation, also referred to as protein "crashing," to remove proteins that might interfere with the separation by fouling the HPLC column. These experiments are performed frequently in 96-well crash plates that contain either 96 one- or two-mL wells or can be a flow-through 96-well filtration plate. Only a small amount (50 μL) of plasma is required, important for small animal studies. The protein is precipitated by the addition of a small volume of acetonitrile, acid, or salt. After agitation, the precipitated protein forms a bead that can be removed by centrifugation or by filtering using a vacuum or positive pressure. The supernatant is then collected, evaporated to dryness, reconstituted in a compatible solvent to 75 μL, and injected into a reversed-phase HPLC column connected to an MS-MS system. The resulting chromatogram (chromatographic conditions not shown), shown in the top on Figure 4b, is quite messy due to the large number of plasma components that were not removed by simple protein precipitation. In addition, the presence of these matrix components in the injected samples also resulted in ion suppression with resulting loss of signal, as evidenced by the intensity of the amitryptyline peak shown at 4.75 min.
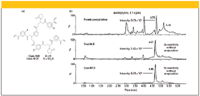
Figure 4: (a) Polymeric packings used in Waters µElution plate. (b) Comparison of protein precipitation and SPE cleanup of plasma using the 96-well-plate format (Oasis µElution plate). The chromatograms are from LCâMS-MS runs using a water-ammonia gradient on Waters Xterra MS column (5). (Courtesy of Waters Corp.)
For the SPE experiments, 96-well flow-through SPE plates were used, which were packed with Oasis (Waters, Milford, Massachusetts) m-divinylbenzene-N-vinylpyrrolidone copolymers. The HLB phase is a neutral polymer while the MCX phase is the same base polymer but derivatized with sulfonic groups, making it a strong cation-exchange packing. Because of the high capacity of the two polymeric packings, only 2 mg of sorbent is packed into each well. This reduced amount of material cuts down on the amount of sample and solvents used for the experiments. The SPE protocol for the Oasis HLB plate involved the conditioning (methanol), equilibration (water), loading (50 μL of rat plasma spiked with 0.1 ng/mL of amitryptyline diluted with 50 μL of water with an added internal standard), washing (5% methanol in water) and eluting (40:60 [v/v] acetonitrile–isopropanol containing 2% formic acid) steps. The SPE protocol for the Oasis MCX plate had the same conditioning, equilibration, and loading steps but the wash step (wash 1: water with 2% formic acid; wash 2: methanol) and the elution step (40:60 [v/v] acetonitrile–isopropanol containing 5% ammonia) were different. Both filtrates were diluted to 75 μL so that they could be compared directly with the protein precipitation experiment. Figure 4b (middle chromatogram) shows the chromatogram observed with the Oasis HLB plate, while Figure 3b (bottom chromatogram) depicts the chromatogram for the Oasis MCX cation exchange method. Note that in both cases, the signal intensity observed for the amitryptyline was significantly higher than for the protein precipitation cleanup (Figure 4b, top chromatogram). In fact, the peak intensity of amitryptyline for the HLB cleanup was 4X the sensitivity of the crash experiment, while the MCX gave 9X the sensitivity (Figure 4b, bottom chromatogram), even with the evaporation step left out for both SPE experiments. The higher intensities undoubtedly were due to the reduction of ion suppression. In addition, the chromatograms were cleaner with interferences substantially reduced compared with protein precipitation. The selectivity of the cation-exchange cleanup was much better than the neutral resin cleanup. Only compounds that showed a positive charged were retained and the rinse step removed many of those compounds that showed up in the HLB extract. This example showed that SPE is still a useful cleanup method even though protein precipitation is simpler. Despite this advantage, workers still prefer to use protein crashing because there is virtually no method development time required, and it relies on the sensitivity and selectivity of the MS-MS system to provide a quick answer.
Molecular Imprinted Polymers
The molecular imprinted polymers (MIPs) are among the most selective phases used in SPE. The technique is sometimes referred to as molecularly imprinted solid-phase extraction (MIP-SPE). Molecular imprinting is a technique that has been used in areas where selective recognition is required for complex separations or sample cleanup. An introductory article (6) outlined the basics of MIP technology, while recent review articles (7–10) and a recent book (11) provide detailed information on the use and potential of MIPs in SPE.
A MIP is a highly stable polymer that possesses recognition sites that are adapted to the three-dimensional shape and functionalities of an analyte of interest. The most common approach through the use of noncovalent imprinting involves the uses of a print molecule (template) that is coupled chemically with one of the building blocks of a polymer. After polymerization, the resulting bond must be cleaved to obtain a free selective binding site (receptor). The selective interactions between the template and the monomers are based upon hydrogen bonding, ionic, and hydrophobic interactions. The most often used monomers are based upon methacrylic acid and methacrylates. The basic idea of an MIP is the "lock and key" concept, in which a selective receptor or cavity on the surface of a polymer perfectly fits the template analyte that was used to prepare the MIP. The receptor site is complementary to the template in terms of its size, shape, and functionality. The concept is similar to immunoaffinity SPE phases but obtaining and linking a suitable antibody for these immunoaffinity sorbents can be very time consuming.
The removal of the template from the polymeric MIP is important not only to make available the interaction sites for increased sample capacity but also to ensure that the analyte to be isolated can be measured quantitatively. The lack of removal of the template molecules, even with exhaustive extraction, has been one of the main problems with the acceptance of MIPs. The template molecules frequently bleed, sometimes give baseline drifts, and interfere with the quantitation of the desired analyte, especially at low levels. One approach to overcome this limitation is to use a template that is similar to the analyte of interest. An example would be to use a brominated analog template rather than a chlorinated molecule of interest. If the analog can be separated from the analyte of interest, then the MIP will function as desired.
With aqueous mobile phases, MIPs can display reversed-phase and ion-exchange interaction because selective polar interactions are impaired. The MIP phases show the greatest selectivity when the experimental conditions are chosen that generate the selective interactions that usually are obtained in organic solvents used for the MIP synthesis. This approach allows the MIP to be used for trapping analytes from aqueous solution by hydrophobic or ionic interactions, then washed with a solvent that breaks selective binding of matrix components, and finally, with an organic solvent which disrupts the strong bonds between the analyte and the MIP polymer matrix.
Because the SPE packing material is a polymer, depending upon the degree of crosslinking, there might be some swelling or shrinkage with a change in solvent. Such a physical change can modify the size of the receptor and change the selectivity of the MIP for the target analyte. Molecular imprinted organic–inorganic hybrid polymers can generate a more rigid substructure that does not swell and shrink.
A disadvantage of the MIP approach to SPE is the fact that each sorbent must be custom made. One determines the specificity of the MIP by choosing the appropriate template molecule. The MIP can be synthesized in the laboratory using published procedures or one can send the template molecule to a specialty laboratory that will make a custom MIP. Because of the relatively long process involved in making an MIP for SPE, one can justify it only if the application will be required frequently or if there is no other way to perform sample cleanup. Recently, off-the-shelf MIPs have been introduced. These standard MIP phases have been designed for specific analytes that are popularly encountered in complex matrices.
Other Polymeric Materials Used in Sample Preparation
In addtion to being used as packings in conventional SPE devices such as cartridges, disks, pipette tips, and 96-well SPE plates, polymers have found use in other sample preparation techniques.
Solid-Phase Microextraction (SPME): This sample preparation technique is nearly two decades old (12). In SPME, an equilibrium method, a solid fused-silica fiber coated with a polymeric stationary phase is placed into a solution or in the headspace of the sample, and analytes diffuse and are moved by convection into the stationary phase. The concentrated analytes are transferred to the chromatography column by thermal desorption (GC) or liquid extraction (LC). The commercial polymers that are coated on the fibers are different than those used for SPE packings. In fact, most of them are GC stationary phases of different film thicknesses such as polydimethylsiloxane (PDMS)-the most popular, polyacrylate (PA), polydimethylsiloxane-divinybenzene (PDMS-DVB), Carboxen-PDMS (CW-PDMS), Carbowax-DVB (CW-DVB) and Carbowax-templated resin (CW-TRP). These phases are available in SPME format from Sigma Aldrich/Supelco (Bellefonte, PA).
The affinity of the polymer coating for an analyte is the most important factor in the successful use of SPME. Selection of the coating is based primarily upon the polarity and volatility of the analyte. Both the coating thickness and distribution constant determine the sensitivity of the method and the extraction time. Thick coatings offer increased sensitivity, but require much longer equilibration times. Therefore, it is important to use the appropriate polymeric coating for a given application (13–15).
Stir-Bar Sorbent Extraction: The amount of PDMS used in SPME is typically in the order of 0.5 μL or less, thereby limiting the enrichment on the PDMS fiber. Based upon this observation, a new approach using stirring bars coated with PDMS was developed (16,17). In this approach, 50–300 μL PDMS coatings are used. Consequently, the sensitivity is increased by a factor of 100 to 1000 (Figure 5). Complete recovery is possible for solutes with kow larger than 500. This technique, named stir-bar sorptive extraction (SBSE), has been applied successfully to the analysis of biological samples (18–21). PDMS-coated stir bars are available commercially under the trade name Twister from Gerstel (Linthicum, Maryland).
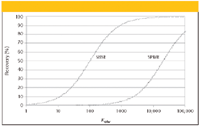
Figure 5: Recovery for solutes in function of the octanol-water partitioning coefficient Kow for SPME (10-mL sample, 100 μm PDMS fiber) and for SBSE (10-mL sample, 10 mm x 0.5 mm PDMS-coated stir bar) (11).
SPE Micropipette Tips with Polymeric Phases: Micropipette tip-based SPE (MPT-SPE) purification, concentration, and selective isolation (affinity, metal chelate) of proteins and peptides is now an essential tool for matrix-assisted laser desorption ionization (MALDI)–MS and for other advanced analytical techniques (22). The first commercially available micropipette tip-based micro SPE (ZipTip) was developed by Millipore (Bedford, Massachusetts) in the late 1990s. ZipTip contains C18 chromatographic media embedded in a polymer. The C18-based ZipTip was used successfully in the purification of peptide mixtures from buffers and salts. In recent years, several manufacturers have developed polymeric MPT products, which can be used for the purification of small amounts of peptides and proteins (23).
Pipette tips with hydrophobic chromatographic media, based upon silica, are available for use in a pH range between 2 and 8 but are unstable beyond this range. Furthermore, they have mostly 300-Å pore sizes and, thus, might not be suitable for larger proteins. POROS RP1 and RP2 (Applied Biosystems, Foster City, California) are polymer-based materials with larger pore sizes (<300 Å) and a wide range of pH resistance and thus are better suited for the purification of certain protein and peptide samples.
Hydrophilic interaction chromatography (HILIC) is a popular technique in HPLC and is useful for the separation of polar compounds that are weakly retained by reversed-phase columns. HILIC polymeric materials can be used in MPT-SPE also. In HILIC, the sample is loaded on the material with 50–90% organic solvent in water, which results in the adsorption of proteins and peptides but not salts and detergents. The analytes are then eluted from the tips with distilled water or 10 mM of a volatile acid such as formic acid. This process can be used for both proteins and peptides. The advantages of this method are that HILIC media do not bind urea or detergents and these impurities can be removed more easily compared to sample preparation based upon hydrophobic interaction chromatographic materials. PolyHYDROXYLETHYL A (PolyLC, Columbia, Maryland) has been available for many years for HILIC sample preparation and separation of peptides and proteins. ProTip, a product from Harvard Apparatus (Holliston, Massachusetts), is based upon an HILIC mechanism. The ProTip is recommended for cleaning and concentrating protein samples before MS. It consists of a hydrophilic polymer that binds protein in presence of organic solvent. The protein is then eluted with aqueous solution. The product has three tip volumes: 1–5, 5–50, and 50–250 μL.
One of the main advantages of using micropipette tips is that they can be used with micropipettors or in liquid handling automation. This has resulted in the routine use of MPT-SPE in bioanalytical labs. It is adapted easily for use in high-throughput screening applications with commercially available xyz liquid handling systems.
Monolith Phases in SPE: A new way to prepare macroporous polymers is by direct polymerization in situ in a mold to produce monoliths. The research group of Frechet and Svec has investigated monolithic materials for SPE (24) based upon PS-DVB and the more polar poly(2-hydroxyethylmethacrylate–ethylstyrene–divinylbenzene) phase. Commercial monolithic-based SPE products in the MPT format have been introduced by Varian (Palo Alto, California) (Omix) and MonoTip from GL-Science (Tokyo, Japan).The Omix products have a small bed of functionalized monolithic sorbent inserted inside a pipette tip. The monolithic SPE phase has no frits and improved flow characteristics compared to packed MPTs. For their standard product, elution volumes are in the range of 2–10 μL; the Omix MB version is mini-bed with elution volumes of 0.5–2.0 μL. Chemistries of C4 and C18 available. The Monotip products have a three-dimensional monolith silica structure with throughpores of 10–20 μm and 20–30 μm and mesopores of 15 and 20 nm, respectively. Volume capacities are 10 and 200 μL. The Monotips are available in individual tips or tips in a 96-well plate rack configuration. Monoliths have promise for microfluidic formats (25) because SPE particles are harder to pack in tiny channels while monoliths can be synthesized in situ.
Immobilized Liquid Extraction: The immobilized liquid extraction (ILE) process involves a device coated with a thin layer of sorptive elastomeric polymer such as PDMS that acts as the extraction medium. A targeted compound is extracted by partitioning into the device's coating from an aqueous solution, the interferences washed away, and the analyte be desorbed (back extracted) in a small volume of organic solvent. The process is reminiscent of SPME, in which a coating is applied to a solid fused-silica tube. Instead, the extraction is performed in a closed system such as the vial where the coating is applied to the cap, on the inner walls of a sealed 96-well plate or on the inner flow path of a micropipette tip. In the vial version (Figure 6), aqueous sample is loaded and agitated while in contact with the extracting surface until analytes have reached equilibrium across the aqueous matrix and the extracting layer. The depleted sample solution is then removed. An optional wash step can follow to remove interferences and matrix components selectively. Finally, the analyte of interest is back-extracted (eluted) from the immobilized phase with an appropriate solvent until equilibrium is obtained. The sample can be injected directly into the chromatograph or blown down and redissolved in a more compatible solvent.

Figure 6: The ILE well plate procedure. (Courtesy of ILE, Ferndale, California.)
Conclusion
Polymeric SPE sorbents are used increasingly in the cleanup and analysis of samples in the pharamaceutical, environmental and food safety areas. They have higher sample capacity, are more rugged, can tolerate accidentally drying out without affecting recovery and reproducibility, and use less solvent than traditional packed-bed, silica-based SPE products. Their spherical shape provides excellent flow characteristics and the absence of silanol groups reduces nonspecific analyte interactions.
The use of polymers in other forms of sample preparation also have seen increased availability. These polymeric phases behave differently than the spherical SPE polymeric packings and are coated on the outside of fused-silica fibers (SPME), on magnetic stirring bars (SBSE) or on the inside of micropipette tips (MPT-SPE), 96-well plates, or vials (ILE).
Ronald E. Majors "Sample Prep Perspectives" Editor Ronald E. Majors is Senior Chemist, Columns and Supplies Division, Life Sciences Chemical Analysis, Agilent Technologies, Wilmington, DE, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com
References
(1) R.E. Majors, LCGC14(9), 754–766 (1996).
(2) R.E. Majors, LCGC15(11), 1008–1015 (1997.)
(3) N.J.K. Simpson, Solid-Phase Extraction: Principles, Techniques and Applications (Marcel Dekker, New York, 2000), p. 6
(4) Technical Note, Polymer Solid-Phase Extraction Cartridges: SampliQ OPT, Agilent Technologies, Santa Clara, California, 2008, Publication Number 5989-8869EN, http://intranet.chem.agilent.com/Library/technicaloverviews/Public/5989-8869EN.pdf
(5) Waters Chromatography Supplies and Columns Catalog, 2008-2009 (Waters, Milford, Massachusetts) Part number: 720002419EN, page 19; www.waters.com/webassets/cms/library/docs/720002419en.pdf
(6) C.L. Arthur and J. Pawliszyn, Anal. Chem.62, 2145 (1990).
(7) J. Pawliszyn, in Applications of Solid Phase Microextraction (Royal Society of Chemistry, Cambridge, 1999).
(8) J. Pawliszyn, J. Chromatogr. Sci.38, 270–278 (2000).
(9) H. Lord and J. Pawliszyn, J. Chromatogr., A885, 153–193 (2000).
(10) E. Baltussen, P. Sandra, F. David, and C. Cramers, J. Microcolumn Sep.11, 737–747 (1999).
(11) F. David, B. Tienport, and P. Sandra, LCGC21(3) 108–118 (2003).
(12) B. Tienpont, F. David, K. Desmet, and P. Sandra, Anal. Bioanal. Chem.373, 46–55 (2002).
(13) T. Benijts, J. Vercammen, R. Dams, H.P. Tuan, W. Lambert, and P. Sandra, J. Chromatogr., B755, 137–142 (2001).
(14) B. Tienpont, F. David, K. Desmet, and P. Sandra, J. Pharm. Biomed. Anal.32, 569–579 (2003).
(15) M. Kawaguchi, K. Inoue, N. Sakui, R. Ito, and H. Nakazawa, J. Chromatogr., B799, 119–125 (2004).
(16) R. Majors, LCGC23(4), 358–369 (2005).
(17) R.E. Majors and A. Shukla, LCGC23(7), 646–660 (2005).
(18) S. Xie, F. Svec, and J.M. Frechet, Chem. Mater.10, 4072 (1998).
(19) C. Yum M. Davey, F. Svec, and J.M. Frechet, Anal. Chem.73, 5088 (2001).

New Study Uses MSPE with GC–MS to Analyze PFCAs in Water
January 20th 2025Scientists from the China University of Sciences combined magnetic solid-phase extraction (MSPE) with gas chromatography–mass spectrometry (GC–MS) to analyze perfluoro carboxylic acids (PFCAs) in different water environments.
SPE-Based Method for Detecting Harmful Textile Residues
January 14th 2025University of Valencia scientists recently developed a method using solid-phase extraction (SPE) followed by high-performance liquid chromatography coupled to high-resolution mass spectrometry (HPLC–HRMS/MS) for detecting microplastics and other harmful substances in textiles.