The Role of Passive Samplers in the Monitoring of Aquatic Ecosystems and Occupational Hygiene Pollution
LCGC North America
Guest authors Luke Chimuka and Ewa Cukrowska provide an in-depth look at the verious approaches to passive sampling, weighing the merits and challenges for each type.
Proper measurement of potential hazards in the environment and in the workplace is important to protect the health and safety of the population. Passive samplers are used in the aquatic and air environment to give a time-weighted average of concentration levels of dissolved pollutants in water and for air toxics as well. Dr Luke Chimuka and Professor Ewa Cukrowska provide an in-depth look at the various approaches to passive sampling, weighing the merits and challenges for each type. They begin by covering the theory, important design aspects, and environmental factors affecting the performance of these samplers. Then, passive samplers used for aqueous and air monitoring are described in detail. Finally, detailing some commercial products, applications examples and competitive techniques round out the coverage.
Monitoring of persistent organic compounds and their derivatives in the ecosystem is a huge and continuing challenge to environmental scientists. In both the aquatic and air environment, it is important to obtain information on the time-weighted average concentrations of pollutants. This gives a better picture of the concentration levels in an ecosystem as it reduces the error caused by short-term concentration variations. Besides, it is important to quantify the concentrations of freely dissolved pollutants in water for approximate estimation of the bioavailable fraction. It is, therefore, important to have an integrative approach, which would provide information about truly dissolved time-weighted average pollutant concentrations over a long time period.
Passive sampling devices allow measurement of an average bioavailable concentration over a long period, on the order of a day to several weeks (1–4). This is as opposed to common sampling methods where total concentrations are measured, including those molecules that are not readily bioavailable because they are bound to dissolved colloids present in environmental media. Information obtained from grab environmental samples is only about concentration levels at the time of sampling and might fail to account for episodic contamination.
Most constructed passive samplers have been based upon membrane extraction technology, mimicking the biological membranes. They typically consist of a receiving phase, with high affinity for organic contaminants, separated from aquatic–air environment by a diffusion limiting membrane (2–4). They are calibrated in the laboratory so the time-weighted average concentrations of organic pollutants can be determined in the field. The area of passive sampling is currently receiving a lot of attention as seen by a number of review papers (5–10). However, most reviews have concentrated either on passive samplers for one particular environmental media (5,8,9) or one particular type of sampler (6,7). Aspects such as commercialization in passive field samplers and potential competitors have not been fully discussed.
Theory
The trapping of chemicals in the passive sampling devices has been described as simple diffusion and partitioning between two compartments of the receiving phase and external environment separated by a diffusing-limiting membrane (4,5,9). The dimensions of the sampling device and the materials it is made of determine the rate of chemical uptake into the receiving phase. In most samplers, the uptake kinetics between a receiving phase and environmental media are described by first-order one compartment mathematical model (5) depicted in Figure 1. This figure gives two main accumulation regimes as either kinetic or equilibrium and these are differentiated during field operation of the sampler. Many passive samplers have been operated in the equilibrium regime such as semipermeable devices (SPMDs) (5). The sampler is deployed long enough so that a thermodynamic equilibrium is established between the chemicals in the environmental media and receiving phase. The first-order equation then reduces to equation 1 (9):
KP = CP/CE (1)
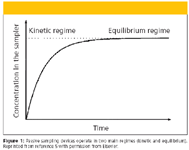
Figure 1
KP is the chemical partitioning between the environmental media (E) and the receiving phase. Cp is the concentration of the trapped chemical in the receiving phase. CE is the concentration of the chemical in the environmental media. However, equation 1 applies to passive samplers with rapid equilibration time of hours to days. These devices are mostly passive samplers with no diffusion limiting boundary layer such as solid-phase microextraction (SPME) and EmporeR (3M, St. Paul, Minnesota) disk-based passive samplers (9). For passive samplers that take time to reach equilibrium, equation 1 has to be modified as discussed by Stuer–Luaridsen (9).
For passive samplers that work in the kinetic regime, it is assumed that the rate of mass transfer to the receiving phase is linearly proportional to the difference between the chemical activity of the contaminant in the environmental media and that in the receiving phase. In this case, the first-order one-compartment mathematical model equation reduces to equation 2 (5):
CP(t) = CE k1 t (2)
Where t is the exposure time, CP (t) is the concentration in the receiving phase after exposure time t. CE is the concentration in the environmental media. k1 is the uptake rate constant. In this case, k1 can be determined by calibration of the sampler in the laboratory. A SPME passive sampler for water sampling has been reported working in the kinetic regime (11). Another sampler based upon silicone hollow fiber membrane called membrane-assisted passive sampler (MAPS) has been reported and works in the kinetic regime too (12).
Important Aspects of Passive Samplers
Sampler design: To have maximum sensitivity, a sampler design should have a high A/L ratio where A is the area and L is the length of the active device. Tube type samplers are therefore less sensitive compared to badge type samplers. The latter have high A/L ratios and most passive samplers are therefore configured in the badge type. Whatever design is employed, passive samplers mostly have a barrier between the sampled medium and the receiving phase. The barrier determines the rate at which analyte molecules are collected in the receiving phase. Some barriers have defined openings resulting into diffusion-based samplers. Others have the barrier in form of a nonporous membrane, referred to aspermeation-based samplers (5). Some factors that influence the uptake rate are sampler design, physicochemical properties of the analytes and environmental variables (for example, water turbulence, temperature, and biofouling) (5).
Quality control: It is very important that the concentration determined using the sampling devices reflect the true picture in the environmental media. Quality control procedures should address issues such as accuracy and precision of the results, contamination, and loss of the trapped analytes.
Stuer-Lauridsen (9) points out that when a passive sampler is retrieved, it must be inspected for signs of puncture, discoloring or any malfunctioning. When passive samplers are calibrated in the laboratory, it is generally easy to obtain good precision (<5%) between replicates. However, in real environmental media, it can be difficult to control certain parameters such as biofouling, turbulence, and temperature. This lack of control results in high standard deviations between replicates and poor accuracy. The precision of some samplers has been reviewed recently (9). In this review, it was noted that the average percentage relative standard deviations for aquatic passive samplers ranged from 10% to 32% (9). The physical chemical properties of the compounds as well the materials used to construct the sampler also can influence the precision of the results similar to active sampling techniques. Others (5,13,14) have suggested the use of permeability reference compounds (PRCs) for quality control. Similar to internal standards, these reference compounds are added to the trapping media before the deployment of the sampler and correct for any changes in uptake rates due to environmental factors. The recovery in reextracting the trapped compounds from the receiving phase also needs to be evaluated as part of the quality control. Recovery is determined by spiking the compounds of interest to the receiving phase and then reextracting them to check for the recovery (14).
Environmental factors affecting passive samplers: Turbulence of the environmental media is one factor difficult to control and can affect the amount of compounds trapped in the receiving phase and therefore the quality of the results. The extent to which turbulence affects uptake kinetics depends upon factors such as sampler material, hydrophobicity of the compound, and environmental flow rates. For membrane-based passive samplers, permeation of compounds through the membrane is seen as the rate-limiting step and is more pronounced for polar compounds (log Kow < 2 where log Kow is the log of the octanol–water partition coefficient) (membrane–permeation-controlled samplers). On the other hand, for nonpolar compounds (log Kow > 3), diffusion through the unstirred layer and sampler controls the mass transfer (donor–diffusional controlled) (15). Once turbulence occurs in the environmental media, the unstirred layer becomes thin and therefore enhances the uptake of nonpolar compounds by the sampler. Depending upon the extent of the turbulence, at very high flow rates, poor dissolution of polar compounds into the membrane can result in decreased uptake rates. PRCs are one way to account for turbulence during sampling (13,14,16). Another way is to enclose the sampler in a container that reduces the effects of turbulence, a technique commonly used in air sampling (1).
In active sampling, it is well known that matrix interferences can influence the accuracy and precision of the extraction process. However, in passive sampling, the main problem is bacteria or other various flora and fauna growing on the surface of the sampler during deployment. This process is called biofouling (2,5). The biofilm formed can affect the sampler in terms of accuracy and precision because these are randomly formed and increase the overall mass transfer resistance. Biofilms also can block the membrane pores in the diffusion-limiting membrane. Richardson and colleagues (17) studied the effect of biofouling on the uptake of trace organic contaminants by SPMDs. The results showed that uptake of contaminants by SPMDs was severely reduced by as much as 50% under fouling conditions compared with unfouled controls. One solution is once more adding PRCs before deployment (13,14,18). Some organic-filled dialysis samplers have been reported to reduce biofouling by slowly seeping out the organic liquid through the membrane (3). Proper choice of the sampler design also can help to reduce biofouling (19).
From kinetic point of view, it is quite clear that temperature of the environmental media can influence the uptake rates in a sampler. A study on the effect of water temperature over a range of 4–20 °C has been reported by Kingston and colleagues (4). In this study, one sampler consisted of a polysulfone limiting membrane while the other had polyethylene. Both of these samplers used the same 47-mm C18 Empore disk as receiving phase. In both cases, an increase in water temperature led to an increase in sampling rate. The effects of temperature on sampling rates have been observed in SPMDs (20) and in membrane-enclosed sorptive coating (MESCO) samplers (21). For practical purposes, it is therefore necessary to determine the effects of temperature in the laboratory for each compound of interest and also measure the temperature during field deployment.
Types of Passive Samplers
Passive samplers for organic pollutants in aqueous environments: There are a number of passive samplers that have been developed for sampling compounds in water bodies. Most of them have been described in recent reviews (5,9). In this article, only a few will be briefly mentioned.
Solvent-filled dialysis bags consist of a dialysis membrane made of regenerated cellulose in the form of a tube filled with an organic solvent, typically hexane. This design was first introduced by Södergren (3). It was the first passive sampler to be introduced for monitoring compounds in water bodies. The selectivity of the sampler is based in differences of the dissolution into the membrane and also on pore size. It was thought to mimic bioconcentration just like in fish and other invertebrates. The dialysis membrane has also a cut off that excludes large molecules, similar to biological membranes. The loss of the organic solvent over time during exposure has been said to prevent biofouling on the surface. The solvent-filled dialysis bag passive sampler has not gained much popularity thus far. However, this sampler is very simple.
The SPMD is perhaps the most common passive sampler in use today. Its design and study was first published by Huckins and colleagues (2). Since then, a number of studies have been performed on them and details can be found in review papers (6,7). SPMDs consist of lay flat tubing made of low-density polyethylene (LDPE) filled with a high molecular weight lipid. Synthetic triolein is often used as the common lipid. The LDPE is nonporous but has transient cavities with a typical size of 1 nm (5). The selectivity of the sampler is based upon the size of the molecules and their ability to dissolve into the membrane. Large macromolecules, ionic compounds, and polar compounds do not dissolve into the membrane. Compounds with log Kow > 3 are ideal for trapping in triolein (7,22). The major shortcomings of SPMDs are the time needed to reextract the trapped compounds from triolein and the use of large organic solvents. Microwave-assisted extraction has been proposed as a faster method to reextract the trapped compounds (20).
Commercially available Empore disks have been used in solid phase extraction technique for extraction of hydrophobic organic compounds in water samples (23). Kingston and colleagues (4) have reported a passive sampling system with Empore C18 disks as receiving phase. In this case, two separate prototype systems have been described, one suitable for the sampling nonpolar organic compounds with log Kow partition coefficient values > 4 and the other for polar species with log Kow values between 2 and 4. Both systems used the same receiving phase but different rate-limiting membranes. The use of well-known and commercially available receiving phase makes this sampler promising. Vrana and colleagues (16) are reported to have calibrated the Chemcatcher passive sampler for monitoring of priority organic pollutants in water. The modified sampler was calibrated for monitoring of hydrophobic micropollutants such as polyaromatic hydrocarbons (PAHs) and organochlorine pesticides in water. Environmental factors such as turbulence and temperature were studied in a flow-through system under controlled conditions. PRCs also were used to correct for environmental factors. The results revealed that the absorption of test compounds on the sampler was similar to their desorption under the same exposure conditions. Therefore, in situ calibration of the sampler is possible using PRCs.
MESCO devices consist of a stir bar coated with poly(dimethylsiloxane) enclosed in a dialysis membrane bag (21). It combines the advantages of passive sampling approach with solventless preconcentration organic solutes from aqueous matrices and subsequent desorption of the sequested analytes on-line with capillary gas chromatography. It avoids cleanup of extracts required for other samplers and whole extract is injected. Injection of the entire extract makes it quite sensitive despite the small surface area and volume of the sampler. The stir bar used as receiving phase is similar to the one used in stir bar sorptive extraction technique (23). The passive sampler was tested for integrative sampling of hydrophobic persistent organic pollutants in the laboratory. Linear uptake rates of all test compounds were observed with one week exposure period (21). MESCO devices recently have been calibrated and tested for field performance for persistent organic pollutants in water (24). Turbulence was evaluated as well as in situ calibration to account for its effects on uptake kinetics. Desorption of chemicals from the sampler was found to be similar to the absorption of the compounds onto sampler under same exposure conditions. This allows for in situ calibration using PRCs. Figure 2 shows the design of the sampler.
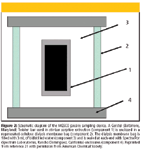
Figure 2
The negligible-depletion SPME approach uses a polymer coating of an optical silica fiber just like in traditional SPME. However, in this case, the fiber is exposed in the headspace above the sample or directly in the sample without any stirring. In the extraction, there is equilibrium between the bound and free fraction of analyte, the depletion of the free fraction is negligible and the binding matrix does not affect the process (25). Negligible-depletion SPME takes advantage of the SPME technique of using low volumes of organic solvents, simple and precise (26). The specific application to measure the free fraction by SPME was introduced by Kopinke and colleagues, and Vae and colleagues (25). Negligible-depletion SPME has the disadvantage of offering only small amounts of the sample for analysis, which can lead to detection limit problems.
Another sampler based upon silicone rubber hollow fiber membrane has been developed (12). The membrane-assisted passive sampling (MAPS) uses a thin walled silicone rubber with dimensions of 0.15 cm (i.d.) × 0.24 cm (o.d.) × 48 cm. In this case, the inside of the tube is filled with an aqueous solution. The tube is sealed on both ends and then is immersed in a water sample. The silicone membrane is hydrophobic and therefore nonpolar organics easily dissolve into it. The pH is adjusted such that the ionizable permeating compounds are ionized and trapped in the aqueous receiving phase. Thus, MAPS uses the same principle as that developed in supported liquid membrane for extraction of ionizable organic compounds (27,28). Its major advantages are its simplicity, low cost, and high selectivity — only ionizable compounds are trapped — and the fact that it uses no organic solvent. Because it is very selective, no further cleanup of the extract is required except possible pH adjustments before injecting the extract into a high performance liquid chromatography (HPLC) system. Its disadvantage is that only ionizable organic compounds can be trapped. However, the silicone rubber traps neutral or uncharged compounds from the water sample. These also can be reextracted from the membrane using organic solvents and analyzed if needed. MAPS has been tested in our laboratory for the passive sampling of chlorophenols and linear uptakes were observed for a 24-h period.
Alvarez and colleagues described the polar organic chemical integrative sampler (29). It consists of a solid receiving phase material enclosed in a microporous polyethersulfone diffusion-limiting membrane. Unlike other samplers with only one type of solid receiving material, this contains a mixture of three solid phase sorbents (Isolute ENV, Argonaut/Biotage, Charlottesville, Virginia, polystyrene–divinylbenzene, and Ambersorb 1500 carbon, Rohm and Haas, Philadelphia, Pennsylvania, dispersed on S-X3 biobeads, BioRad Laboratories, Hercules, California). This combination of sorbents allows the sampler to monitor hydrophilic contaminants such as pesticides, prescription and over the counter drugs, steroids, hormones, antibiotics, and personal care products (29). The sampler has advantages in that it also uses minimal organic solvents and the extracts are analyzed with no further cleanup.
Other passive samplers for monitoring organic compounds in water bodies have been reported. Vrana and colleagues (5) and Stuer-Luarisdsen (9) have highlighted these in their reviews. These include the active carbon–filled acrylic polymer sampler (30), the carbon–filled silicone sampler (31), the silicone sampler with or without resin (18), the ceramic dosimeter (32), and the trimethyl pentane passive sampler, which uses a polymer tube field filled with isooctane as the receiving phase (33,34) and others.
Passive Samplers for Occupational Hygiene (Air): Passive air samplers were introduced about 30 years ago (35). They have been in existence much longer than aquatic samplers. A number of passive samplers can, thus, be found in the literature. Most of the air passive samplers are diffusion based. Recently, the theories of air passive sampling for semivolatile organic compounds have been discussed (36). In most cases, passive samplers for occupational hygiene also can be used for outdoor air sampling. In outdoor sampling, the concentrations of contaminants are much lower than indoor because of dilution. Placing the sampler in a working environment provides air monitoring in occupational hygiene. For small potable samplers, they can be pinned near a worker's breathing zone.
The PerkinElmer (Boston, Massachusetts) sampler consists of a stainless tube with silicone membrane at one end (10). The tube is then filled with appropriate sorbent for trapping the compounds. Figure 3 shows the design of the sampler (10). The type of compounds to be trapped determines what sorbent material to use (37,38). The trapped compounds can be thermally desorbed into the gas chromatography.
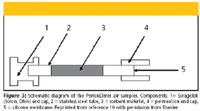
Figure 3
The polyurethane foam (PUF) sampler uses polyurethane, in form of a disk, as a trapping material that is housed in stainless steel chambers. The chambers dampen wind speed effects and protect the sampling medium from coarse deposition (39,40). The sampler is also not very complicated. However, the trapped compounds have to be re-extracted usually with Soxhlet extraction that is time consuming and uses a large amount of organic solvent. Shoeib and Harner (40) characterized and compared the PUF passive sampler with SPMDs (see the following). Comparable results were observed between the two samplers for monitoring of persistent organic pollutants in air.
SPMDs, discussed earlier, are versatile and rugged in that they can be used to monitor both air and aquatic environment (13). Ockenden and colleagues (41) studied the effects of wind speed on the uptake rates and the use of PRCs in air sampling. The results indicated that if SPMDs are deployed in Stevenson screens under ambient conditions, wind speed does not significantly affect uptake rates. However, the temperature was observed to affect uptake rates especially when deployed over different seasons (winter–summer). Results also indicated that the use of PRCs could correct influence of environmental factors. The applicability of SPMDs as air samplers for lipophilic organic contaminants from vapor phase has been studied by Petty and colleagues (42), who found out that they could concentrate polychlorinated biphenyls (PCBs) from laboratory atmosphere in a linear manner over a 28-day period. Trapped analytes, just like in aquatic environments, have to be re-extracted, which requires time and use of organic solvents. So far not many applications have been made to monitor pollution in workplaces.
SKC Inc. (Eighty Four, Pennsylvania) (43) has a number of passive samplers for monitoring volatile organic compounds in air. These contain different sorbents for trapping a variety of contaminants. For trapping formaldehyde in work places, the sampler (UMEx 100) uses 2,4-dinitrophenyl hydrazine to produce an aldehyde derivative. Another SKC sampler is the badge type (10). This badge-type passive sampler consists of a sorbent that traps the target compounds that pass through a diffusive barrier. The sampler has a desorption solvent chamber that allows for easy removal of the trapped compounds.
There are various other passive samplers for indoor and outdoor air monitoring. Some of these are highlighted in the following paragraphs.
The Ogawa passive sampler has been described by Roadman (44). It consists of a sampler body with independent cavities that have diffusive barriers. The trapping material is a reactive filter impregnated with inner and outer stainless steel screens (45). It can be used for trapping gases such as NO, NO2, NOx, O3, SO2, and NH3 (45).
The air samplers that can monitor organic vapors referred to as 3M 3500 and 3M 3520 (3M, St. Paul, Minnesota) have been reported (46,47). These use charcoal sorbents for trapping potential contaminants. The 3M 3520 air sampler has two wafers of charcoal and is therefore suited for collecting compounds at unknown concentrations where the capacity of the 3M 3500 monitors can be exceeded. Desorption of the trapped compounds is done by adding an appropriate solvent to the charcoal pad.
Another small prototype passive air sampler that uses a carbonaceous adsorbent disk has been described (48). The adsorbent material is contained in a glass insert housed in amber glass vial with a screw and filter at the top. It has been evaluated for monitoring of volatile organic compounds (48). The trapped compounds are reextracted using a suitable solvent such as CS2. The sampler is quite simple.
Cruz and colleagues (49) constructed a passive sampler for monitoring SO2 in industrial and urban air based upon molecular diffusion. SO2 is trapped in impregnated filters with Na2CO3. The trapped gas is later extracted in an ultrasonic bath using H2O2 as solvent. SO2 in this case is determined as SO42- using ion chromatography.
Polymer-coated glass and polyethylene-based samplers have been reported by Harner and colleagues (50) and Bartow and colleagues (51), respectively. The polyethylene-based sampler is suited for collecting semivolatile compounds and has been characterized by studying the uptake kinetics of PAHs from air. However, the successful operation requires a great deal of time and organic solvent, usually hexane, to reextract the trapped compounds. The polymer-coated glass sampler was tested for the collection of PCBs in the gas in an indoor environment. The polymer material in this case was ethylene vinyl acetate (EVA). The uptake kinetics was similar to any passive sampler. The sampler was affected by wind speed but when housed in deployment chambers consisting of inverted stainless steel bowls, the effects were diminished. The EVA-based sampler also requires time and organic solvents to re-extract the trapped compounds from the polymer.
Competitive Techniques to Passive Samplers
Active sampling: Active sample preparation techniques such as liquid–liquid extraction (52), solid-phase extraction (53), and SPME (26,54) are still the major competitors to passive sampling, especially for monitoring compounds in aquatic ecosystems. These extraction techniques are well developed and known to provide very good accuracy and precision (typically below 5%). Because extraction is performed in the laboratory, environmental factors that affect passive samplers are easily controlled. Quality assurance procedures of these techniques are also well known. On other hand, quality assurance procedures for passive sampler especially for monitoring aquatic ecosystems are still being developed. Sample preparation is generally known to consume 60% of the environmental monitoring process, and most errors are introduced during this step (55). Using passive samplers therefore makes much more sense because sampling and sample preparation are combined in one step. This advantage is especially true for the passive samplers whose extracts do not need any further clean up. Passive samplers can easily be deployed over large area because they need no pumps and are much cheaper. In the near future, after quality assurance procedures become well known, passive samplers should become a more attractive alternative to common active sampling and sample preparation techniques.
Biomonitoring organisms: Biomonitoring organisms have been used as passive samplers and have the major advantage that they reflect the true impact of the condition of the environment (10). They do not need any deployment and preconcentrate the compounds through bioconcentration. However, they have some limitations too in their use as passive samplers. Biomonitoring organisms cannot survive in certain environmental conditions and age, size, sex, and physical condition might affect the uptake rates of compound (56). The organisms should be abundant and for long-term monitoring should be less mobile in the environment (10). The trapped compounds also need to be re-extracted and often require additional clean-up step. A number of biomonitoring organisms have been reported and compared with SPMDs with good correlation observed in most cases (14,57,58).
Commercial Passive Samplers
Commercialization of the passive sampler is very important because it reflects acceptance by the wider community. This means that potential users know where to buy the sampler with standard dimensions. It then becomes easy to compare the results and also to use the same sampling rates for the same compounds. This reduces the time needed to calibrate the sampler in the laboratory before deployment. Table I shows some of the commercial suppliers of passive samplers. Very few passive samplers for aquatic ecosystems have been commercialized. However, many of them have patent protection. A number of samplers for aquatic ecosystem are just being developed or validated. Research in developing samplers for water bodies is on the increase (5) and in the near future many new samplers will be commercially available. On the other hand, many air passive samplers are commercially available.
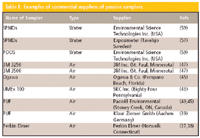
Table I: Examples of commercial suppliers of passive samplers
Applications of Passive Samplers
Aquatic ecosystems: Detailed applications of the passive samplers for aquatic ecosystem monitoring have been reviewed by Vran and colleagues (5) and Stuer–Laurisden (9). About 76% of the applications have used SPMDs (5). This is not surprising because many other passive samplers are new having just been developed (12) or calibrated (16,24). Various compounds have been monitored by passive samplers especially using SPMDS including pesticides and biocides, organochlorines and organohalogens, and aromatic and alkylated aromatic compounds (9).
A review on the background and application of the SPMDs in aquatic ecosystems has been published (6). Verwei and colleagues (14) assessed the bioavailable PAH, PCB, and organochlorine pesticide concentrations in fresh water sites in and around the city of Amsterdam using SPMDs. The study also compared the PAH concentrations in SPMDs to those found in sediments and caged carp. A significant correlation was observed between biliary PAH metabolite levels in fish and aqueous concentrations estimated with SPMDs. For quality control and assurance, SPMDs were spiked with standard solutions of PAHs, PCBs, and organochlorine pesticides in hexane. The recovery in this case was 92–110% for PAHs, 105–115% for PCBs and 74–82% for nonpolar pesticides. SPMDs also were used to assess the level of pollution in Lithuania (61). Predictions of aqueous concentrations were done in the linear uptake rates region for eight pesticides of different classes (organochlorines, synthetic pyrethroids, dinitroanilines, and amides) over a 20-day exposure. In this case, accurate water concentrations did differ with estimated concentrations from SPMDs. Bioassays also were incorporated in SPMDs to assess the effects of concentrated pesticides.
Occupational hygiene (air): Most of the air passive samplers are currently applied to outdoor environments. This widespread application can, due to general concerns that levels of atmospheric pollution are on the increase. Application of passive samplers in the workplace is driven by concerns that workers' lives could be in danger due to the pollutants emitted. A few will be highlighted here.
A number of applications have been made on the monitoring of air pollution in work places using the PUF disk sampler (1,39,62). Polybrominated diphenyl ether (PBDE) flame retardants have been monitored in indoor and outdoor air on Ottawa, Canada using PUF disk samplers (1). Indoor air concentrations of PBDEs were found to be 50 times higher than outdoor air concentrations. Flame retardants also were monitored in indoor air at a electronics recycling plant and other work places in Sweden using PUF disk samplers. Many flame-retardants were detected in all indoor air with highest concentration being detected from the recycling plant. Flame retardants also have been monitored using PUF in Kuwait (39). These compounds seem to be of interest because of their volatilization from foam (for example, mattresses and foam-padded furniture), electronic equipment, and other products to which they are added as flame retardants (39).
The PerkinElmer passive sampler has been used to monitor volatile organic compounds and particulate bound PAHs inside and outside a Baltimore (Maryland) Harbor Tunnel tollbooth (63). This study was conducted to assess the exposure of tollbooth workers to these compounds. Concentrations of benzene and 1,3-butadiene were highest outside the tollbooth compared with the inside as might be expected because the major source is vehicles passing the tollbooth. However, the concentrations of chlorinated hydrocarbons were observed at high concentrations within the tollbooth indicating an indoor source and opportunity for exposure mitigation. Patil and Lonker (38) have determined and compared benzene, aniline, and nitrobenzene levels in work places using PerkinElmer samplers. Their method is said to give precise and accurate results that are within the National Institute for Occupational Safety and Health (NIOSH) acceptability criteria of 25%.
Another study used 3M 3500 air samplers to assess personal exposure to 1,3-butadiene and styrene in three plants manufacturing styrene–butadiene copolymers. In this case, air samples were collected from the breathing zone. The method is said to be simple and inexpensive.
Future trends
Research in developing and applications of passive samplers especially for aquatic ecosystems is on the increase (5). Future trends could be in the following areas:
- Improving the quality assurance for estimation of concentrations in the environment by incorporating performance reference compounds.
- Incorporation of bioassays in the trapping media to rapidly assess the toxicity of the trapped compound concentrations.
- Using trapping materials that minimize the need to re-extract the trapped analytes.
- Developing trapping materials and samplers that can trap polar organic compounds and metal ions.
Dr. L. Chimuka and Prof. E.M. Cukrowska are with the School of Chemistry, Environmental analytical chemistry research group, University of the Witwatersrand, Johannesburg, South Africa. Please direct correspondence to Luke@chem.wits.ac.za
Ronald E. Majors
"Sample Prep Perspectives" Editor Ronald E. Majors is business development manager, Consumables and Accessories Business Unit, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgc-mag.com

Ronald E. Majors
References
(1) B.H. Wilford, T. Harner, J. Zhu, M. Shoeib, and K.C. Jones., Environ. Sci. Technol. 38, 5312 (2004).
(2) J.N. Huckins, M.W. Tubergen, and G.K Manuweera., Chemosphere 20, 533, (1990).
(3) A. Södergren, Environ. Sci. Technol. 21, 855 (1987).
(4) J.K. Kingston, R. Greenwood, G.A. Mills, G.M. Morrison and L.B. Person., J. Environ. Monit. 2, 487 (2000).
(5) B. Vrana, G.A. Mills, I.J. Allan, E. Dominiak, K. Svensson, J. Knutsson, G. Morrison, and R. Greenwood, Trends Anal. Chem. 24, 845 (2005).
(6) Y. Lu, Z. Wang, and J. Huckins, Aquatic Tox. 60, 139 (2002).
(7) J.D. Petty, C.E. Orazio, J.N. Huckins, R.W. Gale, J.A. Lebo, J.C. Meadows, K.R. Echools, and W.L. Cranor, J. Chromatogr. A 879, 83 (2000).
(8) A. Kot, B. Zabiegala, and J. Namiesnik, Trends Anal. Chem. 19, 446 (2000).
(9) F. Stuer-Lauridsen, Environ. Pol. 136, 503 (2005).
(10) T. Gorecki and J. Namiesnik, Trends Anal. Chem. 21, 276 (2002).
(11) G. Ouyang, Y. Chen, and J. Pawlisyzn, Anal. Chem. 77, 7319 (2005).
(12) L. Chimuka, E. Cukrowska, T. Nemutanfani, and N.A. Masevhe, "Development of novel membrane assisted passive sampler based on silicone rubber." submitted to Water Research Commission, (2006)
(13) K. Booij and B.L. Van Drooge, Chemosphere 44, 91 (2000).
(14) F.Verweij, K. Booij, K. Satumalay, and N. Van der Molen, Chemosphere 54, 1675 (2004).
(15) N. Mergesa, L. Chimuka, T. Solomon, and J.A. Jonsson, J. Sep. Sci. 24, 567 (2001).
(16) B. Vrana, G.A Mills, E. Dominiak, and R. Greenwood, Environ. Pol., in press.
(17) B.J. Richardson, P.K.S. Lam, G.J. Zheng, K.E. McClellan, and S.B. De Luce-Abott, Marine Pol. Bulletin 44, 1372 (2002).
(18) K. Booij, F. Smedes, and E.M. Van Weerlee, Chemosphere 46, 1157 (2002).
(19) J.D. Petty, J.N. Huckins, D.A. Alvarez, W.G. Brumbaugh, W.L. Cranor, R.W. Gale, A.C. Rastall, T.L. Jones-Lepp, T.J. Leiker, C.E. Rostad, and E.T. Furlong, Chemosphere 54, 695 (2004).
(20) V. Yusa, A. Pastor, and M. de la Guardia, Anal. Chim. Acta 540, 355, (2005).
(21) B. Vrana, P.Popp, A. Paschke, and G. Schuurmann, Anal. Chem. 73, 5191 (2001).
(22) J.N. Huckins, J. Petty, J.A. Lebo, C.E. Orazio, H.F. Prest, D.E. Tillit, G.S. Ellis, B.T. Johnsson, and G.K. Manuweera, Techniques in Aquatic Toxicology (Lewis CRC Press, Boca Raton, Florida, 1996).
(23) E. Baltussen, P. Sandra, F. David, and C. Cramels, J. Micro Column Separations 11, 737 (1999).
(24) B. Vrana, A. Paschke, and P. Popp, Environ. Pol., in press.
(25) M. Heringa and J.L.M Hermens, Trends Anal. Chem. 22, 575 (2003).
(26) C.L. Arthur and J. Pawliszyn, Anal. Chem. 62, 2145 (1990).
(27) L. Chimuka, N. Megersa, J. Norberg, L. Mathiasson, and J.A. Jonsson, Anal. Chem. 70, 3906 (1998).
(28) J.A. Jonsson and L. Mathiasson, Trends Anal. Chem. 18, 325 (1999).
(29) D.A. Alvarez, J.D. Petty, J.N. Huckins, T.L. Jones-Lepp, J.P Goddard, and S.E. Manahan, Environ. Tox. Chem. 23, 1640 (2004).
(30) F.A. Digiano, D. Elliot, and D. Leith, Environ. Sci. Technol. 22, 1365 (1989).
(31) G.Z. Zhang and J.K. Handy, J. Environ. Sci. Health A 24, 279 (1989).
(32) H. Martin, B.M. Pa Herson, and G.B. Davis, Environ. Sci. Technol. 37, 1360 (2003).
(33) J.M. Zabik, L.S. Aston, and J.N. Seiber, Environ. Toxicol. Chem. 11, 765 (1992).
(34) S.M. Peterson, S.C. Apte, G.E. Batley, and C. Coade, Chem. Spec. Bioavail. 7, 83 (1995).
(35) E.D. Palmes, A.F. Gunniston, J. DiMathio, and C. Tomcyk, American Ind. Hygiene Assoc. Journal 37, 570 (1976).
(36) M.E. Bartkow, K. Booij, K.E. Kennedy, J.F. Muller, and D.W. Hawker, Chemosphere 60, 170 (2005).
(37) J. Ballach, B. Greuter, E. Schultz, and W. Jaeschke, Sci. Total Environ. 243–244, 203 (1999).
(38) S.F. Patil and S.T. Lonkar, J. Chromatogr. A 688, 189 (1994).
(39) B. Gevao, M.A. Bahloul, A.N. Al-Ghandban, L. Ali, A. Al-Omair, M. Helaleh, K. Al-Matrouk, and J. Zafar, Atmospheric Environ. 40, 1419 (2006).
(40) M. Shoeiba and T. Harner, Environ. Sci. Technol. 36, 4142 (2002).
(41) W.A. Ockenden, B.P Corrigan, M. Howsam, and K.C. Jones, Environ. Sci. Technol. 35, 4536 (2001).
(42) J.M. Petty, J.N. Huckins, and J.L. Zajicek, Chemosphere 27, 1609 (1993).
(43) SKC Inc., Eighty Four, Pennsylvania.
(44) M.J. Roadman, J.R. Scudlark, J.J. Meisinger, and W.J. Ullman, Atmospheric Environ. 37, 2317 (2003).
(45) Ogawa & Co., Pompano Beach, Florida.
(46) T.A. Klemetti, R. Vaaranrinta, P. Mutanen, and K. Peltonen, Int. J. Hyg. Environ. Health 209, 151 (2006).
(47) 03M Australia, automotive and marine.
(48) R. Otson and X.L. Cao, J. Chromatogr. A 802, 307 (1998).
(49) L.P.S. Cruz, V.P. Campos, A.M.C. Silva, and T.M. Tavares, Atmospheric Environ. 38, 6425 (2004).
(50) T. Harner, N.J. Farrar, M. Shoeib, K.C. Jones, and F.A.P.C. Gobas, Environ. Sci. Technol. 37, 2486 (2003).
(51) M.E. Bartkow, D.W. Hawker, K.E. Kennedy, and J.F. Muller, Environ. Sci. Technol. 38, 2701 (2004).
(52) J.R. Dean, Extraction Methods for Environmental Analysis (John Wiley and Sons, Chichester, 1998).
(53) C.F. Poole and I.D. Wilson, J. Chromatogr. A 885, 1+2 (2000).
(54) J. Beltran, F.J. Lopez, and F. Hernandez, J. Chromatogr. A 885, 389 (2000).
(55) R. Majors. LCGC 10(4), 282–293 (1991).
(56) D.T.H Phillips, Quantitative Aquatic Biological Indicators: Their Use to Monitor Trace Metal and Organochlorine Pollution (Applied Science Publ., London, UK, 1980).
(57) C. Goarlay, C. Miege, A. Noir, C. Ravelet, J. Garric, and J.M. Mouchel, Chemosphere 61, 1734, (2005).
(58) B.J. Richardson, G.J. Zheng, E.S.C. Tse, and P.K.S. Lam, Chemosphere 45, 1201 (2001).
(59) Environmental Science Technologies Inc.
(60) Pacwill Environmental Co.
(61) P. Sabaliunas and A. Sodergren, Environ. Pol. 96, 195 (1997).
(62) A. Sjodin, H. Carlsson, K. Thuresson, S.Sjolin, A. Bergman, and C. Ostaman, Environ. Sci. Technol. 35, 448 (2001).
(63) A. Sapkota, D. Williams, and T.J. Buckley, Environ. Sci. Technol. 39, 2936 (2005). (64)

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









