Interpretation of Mass Spectra, Part I: Developing Skills
LCGC North America
In this column, Michael Balogh proposes some practices that you can adopt to enhance your prospects of being right when converting data to knowable information and applying it to solving problems.
In the last decade, the trend toward coupling mass spectrometers to every delivery system imaginable has so burgeoned that we now find ourselves challenged to accurately interpret the torrent of data that stream from new, highly evolved systems. Those data are being cached and archived far more efficiently than they were even a few years ago, when local optical disks were considered hot technology. But every path has a puddle, and converting the data to knowable information and applying it to solving problems still proves a difficult, often angst-producing art.
Certainly, sophisticated software programs that supplement spectral interpretation have been developed. Yet, looking your colleagues in the eye and feeling secure in the accuracy of your results as you hand them over still requires technical skills. In this month's column, we propose some practices that you can adopt to enhance your prospects of being right.
As we discussed in a previous column, you can subscribe to online resources that over time will undoubtedly increase your general understanding. But chances are that they will not address your need for an immediate answer when a question arises unexpectedly. Then there are consultants, who can be expensive. But when their efforts are well directed, consultants can be indispensable. Perhaps rule number one, though, should be: choose your instrument provider well. In the recent past, many manufacturers of analytical instruments have merged with, been acquired by, or acquired other companies in their industry. As a result, many companies now design, produce, and market both high performance liquid chromatography (HPLC) and mass spectrometry (MS) instruments. Such consolidation should be to the consumer's benefit: you should receive "system-level" support when questions arise, not an LC-centric or MS-centric answer. So a manufacturer's training, sales, and service support staffs can be a good first line of defense against finding yourself stymied by a spectrum that thwarts your best interpretive efforts.
You also might seek advice from someone offering in-service training. Through his persistence and diligence, O. David Sparkman, (University of the Pacific, Stockton, California) has earned a reputation as the "dean of continuing education in mass spectrometry." For longer than I have been involved with MS, he has been active as a consultant and educator, providing ACS courses, authoring books and reviews, and adding his comments to online MS message board discussions. David has added some of his thoughts to this month's topic and you can reach him directly at ods@csi.com
The mass spectrometer's data output is the result of our evolving ability to detect ions in a vacuum, an evolution that began many years ago when electronics were analog and displays were oscilloscopes. The retro Diehls–Alder reactions and hemolytic/heterolytic energies required to disassociate or cleave bonds leading to specific well-characterized fragmentation continues to be the basis for our thinking when confronted with mass spectra.
The difficult part of MS is often in answering the question: "What is the mass we are dealing with?" Until the development of desorption techniques like matrix-assisted laser desorption ionization (MALDI) and electrospray, that question was a much easier one to answer. MS dealt with the m/z value of ions displaying only a single charge. The mass of an analyte usually was reported as the nominal mass (or nominal m/z value) of the molecular ion, the same as the nominal mass of the molecule. The nominal mass of an ion, molecule, or radical is the sum of the nominal masses of the elements in its elemental composition. The nominal mass of an element is the integer mass of the most abundant naturally occurring stable isotope; sometimes referred to as the principal isotope. But the answer became more elusive when soft-ionization desorption techniques like electrospray ionization (ESI) became commercially widespread beginning in the early 1990s. In the "pre-desorption" age of MS, the nominal mass of most analytes interrogated by MS was less than 500 Da. Mass defect due to the presence of hydrogen was not an issue for these analytes. The negative mass defect for halogenated species was a possible problem only for compounds that contained more than three atoms of bromine. The upper m/z limit for most mass spectrometers fell in the 650–800 range. Thus, in those pre-desorption ionization days, the nominal mass and the integer monoisotopic mass were the same value. The monoisotopic mass of an ion, molecule, or radical is the sum of the monoisotopic masses of the elements in its elemental composition. The monoisotopic mass of an element is the exact mass of the most abundant naturally occurring stable isotope; sometimes referred to as the principal isotope.
But at the onset of the desorption ionization era, larger molecules and greater precision became integral to studies because the technology permitted it with little difficulty. It was then that the issue of mass defect became very important. In a mass spectrometer able to report only to the nearest integer m/z value, the molecular ion of a C50H102 compound could be represented by a peak at m/z 703 instead of at m/z 702. That is because the molecular ion of C50H102 would have a monoisotopic mass of 702.7825, which rounds to the integer 703. The molecular ion of C6Br6 could be represented by a peak at m/z 545 instead of m/z 546 if a hydrocarbon mass defect correction (100 mmu per 100 u) was applied to the monoisotopic mass of 545.5100 before rounding to the nearest integer.
Above 500 Da, mass defect can be a serious issue in determining the m/z values of MS peaks. It is important to keep in mind that the mass spectrometer is measuring signal intensities that occur at a specific time during the collection of the mass spectrum, regardless of the type of m/z analyzer used. The m/z value reported is a function of the time that ions of a known m/z value produced by a specific compound — relative to the calibration compound — reach the detector. Because the mass of monoisotopic ions changes as the position on the m/z scale changes, the mass spectrometer that reports integer m/z values actually can take measurements at every 0.05 m/z units. The detected intensity can be that at the apex of the mass spectral peak or the sum of the intensities across the mass spectral peak. The m/z value reported is an integer obtained by rounding the observed m/z value for the mass spectral peak maximum.
Electron ionization MS often relies on perfluorinated compounds like perfluorotributylamine (nominal molecular mass = 671) to calibrate the m/z scale. That is because the integer mass of an ion is almost the same as its monoisotopic mass (monoisotopic mass of PFTBK = 670.9600). Once an ion exceeds a nominal mass of 1000 Da, there will be no observed nominal m/z value peak in the mass spectrum. The monoisotopic mass peak will be offset from where the nominal mass peak should be observed by an amount equal to the mass defect of the ion. Above 1000 Da, there will be no observed nominal m/z peak value in the mass spectrum.
For single-charge ions with masses above 500 Da, using techniques such as electrospray in combination with transmission quadrupole or quadrupole ion trap mass spectrometers that have unit resolution throughout the m/z scale, the isotope peaks will be separated clearly. That is, the separation of ions that have m/z 3500 and 3501 is the same as the separation of ions that have m/z 100 and m/z 101. However, for an instrument with a constant resolving power (R) (meaning that the value for M/Dm is a constant), the mass spectral peak can yield a full-width-at-half-maximum value greater than a single m/z value.
Consider the case in which a peak represents a single-charge ion at m/z 200,000 on a MALDI time-of-flight (TOF) mass spectrometer with a resolving power of 2000. The full width-at-half-maximum value for the peak at m/z 200,000 would be 100 m/z units. So what mass does the peak represent? It cannot be a nominal mass. Nor can it be a monoisotopic one. It is, however, a mass derived from the weighted average of all the masses in the range of m/z 199,950 to m/z 200,050, a weighted average based upon the abundance of each ion in the range. The effect is the same as it would be if the mass of the ion were calculated using the weighted average of the naturally occurring isotopes that make up the ion — that is, the atomic weights (atomic masses) of the elements. The symbol used for this "average mass" is Mr.
Figure 1 provides an example of a useful series of graphics that explain the effect of increased instrument resolution on peak characterization. The graphics appear in An Overview of Peptide and Protein Analysis by Mass Spectrometry, S. Carr and R. Annan, in Current Protocols in Protein Science (J. Wiley and Sons, 1996).
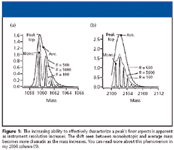
Figure 1
In a mass spectrometer with high resolving power, the m/z value of a peak represents a weighted average of ions. This is true even for ions of low m/z value due to high masses and a multiplicity of charge states because the numeric separation of the isotope peaks is far less than one m/z unit. So we can no longer blithely state that MS deals with the mass of the isotopes of elements, not their atomic weights.
In Sparkman's view, interpretation of mass spectra obtained through collisionally activated dissociation (CAD), also known as collision-induced dissociation (CID), is no different from interpreting mass spectra obtained via electron ionization or any other ionization technique. It should be noted in our discussion here that this broad statement is useful where otherwise, in specific cases, caveats should be noted.
First, look for an ion (peak) in a spectral display that represents the intact molecule: If the spectrum in question is a product of atmospheric pressure ionization (APCI) or ESI mode, look for ions that represent the protonated (M + H) or deprotonated (M - H) molecule. You must take into account the possibility of adduct ions forming with solvent and other molecules and ions endogenous to the sample and matrix. Make sure the isotope peaks are separated by one m/z unit, a good sign the peaks represent a single-charge ion.
Apply the nitrogen rule to determine whether the analyte has an odd or even number of nitrogen atoms: If the molecular mass of the analyte is greater than 500 Da, don't forget about mass defect. Use the intensity of isotope peaks to gain additional information about the elemental composition of the precursor ion and fragment ions. It is good practice to include a range of m/z values when selecting the precursor ion so that you can follow the isotope patterns in the decomposition. If available, use accurate mass measurements to determine the elemental composition of the precursor ion and its fragments. Even with accurate mass measurements, don't ignore the relative intensities of isotope peaks. In a future column, we will explore this point in greater depth because software programs increasingly take effective advantage of unique features such as isotopes in the spectrum, that appear in high fidelity on modern mass spectrometers.
Classic Electron Ionization-based Algorithms
An elemental composition can be used in an empirical formula search against the NIST/EPA/NIH Mass Spectral Database of electron ionization (EI) spectra (in the NIST Mass Spectral Search Program). Many common compounds found when analyzing an unknown by APCI and ESI are also in the EI database. The formula search can yield a lengthy list of compounds, or it might yield a single "hit," as with C12H18C12N2O. The only compound in the NIST05 EI database with that elemental composition is clenbuterol. Bear in mind that, as I discussed in a previous column (2), the Journal of the American Society of Mass Spectrometry has promulgated rules that specify how to determine an unambiguous formula when you identify a compound based upon accurate mass results.
Examine the list of matches for multisynonymous compounds and for those found in other databases, like the ones maintained by the United States Environmental Protection Agency (U.S. EPA) and National Institutes of Health (NIH). Inclusion in as many as nine different databases is part of each entry in the NIST05 database. Compounds in the list of possible candidates with synonyms and those included in other databases are more likely to be the unknown than those without either of these attributes. Compounds lacking synonyms or those not found in other databases are far less commonly encountered and usually originate as one-time-synthesis results.
If an elemental composition results from accurate mass, a simple test of all the possible compositions using the NIST MS Search Program's formula search can be performed. You also can conduct a molecular weight search in the NIST MS search program, using the nominal mass of the unknown as you would a formula search.
Look at the differences in the m/z values of the precursor and product ions: Differences in the m/z values between the precursor and product ions represent the dark matter — the neutral losses — in MS. When a molecule fragments in MS, by whatever means, not all fragments become charged, and those that do not are lost to detection. Keep in mind the even-electron rule: "The decomposition of even-electron ions is strongly influenced by the preference for the formation of an EE+ ion and an EE0 neutral." This phenomenon holds especially true for low-energy MS–MS in quadrupole ion traps (QIT) and collision cells using triple-quads or hybrids. Rearrangements dominate, which can be problematic for the interpreter.
Look carefully at mechanisms associated with hydride-shift rearrangements of EE+ fragment ions. Such rearrangements are formed by decomposing molecular ions produced by electron ionization from aliphatic alcohols and amines. These same-type rearrangements often are seen with protonated nitrogen and oxygen atoms in CAD reactions.
In a subsequent column, we will continue examining spectral interpretation issues, taking a close look at software programs: how they can be applied and how a practitioner can avoid the overconfidence that leads to incorrect answers. The programs have become quite seamless in their user interface, and their efficacy has improved.
Most people who generally succeed when determining structures of analytes from spectra obtained by CAD are well-versed in interpreting EI mass spectra. Fragmentation of an ion always occurs by the breaking of chemical bonds and the loss of neutral species that conform to valence rules. Fred McLafferty's "red book," developed and revised over many years (depending upon the edition, it might indeed be another color) constitutes the single best source of information detailing the variety of energetics involved in MS analysis (3). Its many isotope tables provide a well-characterized platform for interpreting spectra. In the end, however, McLafferty's words on the subject are most memorable: "All you are dealing with is the mass of the molecule and the masses of the pieces of the molecule."
"MS — The Practical Art" Editor Michael P. Balogh is principal scientist, LC–MS technology development, at Waters Corp. (Milford, Massachusetts); an adjunct professor and visiting scientist at Roger Williams University (Bristol, Rhode Island); and a member of LCGC's editorial advisory board.
References
(1) M.P. Balogh, LCGC 22(2), 118–130 (2004).
(2) M.P. Balogh, LCGC 24(2), 140–148 (2006).
(3) F.W. McLafferty and F. Turecek, Interpretation of Mass Spectra, 4th edition, (University Science Books, Mill Valley, California, date TK).
Erratum
David Bostwick of the Georgia Institute of Technology points out that the correct name of the website listed in the April installment of "MS-The Practical Art" (M.P. Balogh, LCGC 24[4], 384 [2006]) should be sci.techniques.mass–spec.
He adds, "I've enjoyed reading your column, and even with 30 years of MS experience, I get a refresher course or a new idea from them."

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










