The Role of Liquid Chromatography and Gas Chromatography in the Analysis of Illegal Medicines and Health Products
LCGC Europe
This review article will give a general overview of the liquid chromatographic (LC) and gas chromatographic (GC) methods used by analytical laboratories for the detection and characterization of suspected illegal medicines and health products, including lifesaving drugs (antimicrobials and antimalarials), lifestyle drugs (erectile dysfunction drugs), and biotechnology drugs (doping peptides and skin-tanning peptides). Literature published from 2015 until early 2019 will be surveyed.
Falsification or adulteration of medicines and health products is a long-standing issue posing a serious menace to public health. These substandard and falsified (SF) medical products could result in treatment failures, drug resistance, and serious adverse drug reactions. In response to the increasing concern about the occurrence of falsified medical products in the European Union, the Falsified Medicines Directive 2011/62/EU will be implemented in the beginning of 2019. This directive creates a preventive system for falsified medicines. Alternatively to the global regulatory efforts that are being made, regulatory authorities, including official medicine control laboratories (OMCL), also need efficient analytical methods to monitor drug quality and survey the (illegal) market. This review article will give a general overview of the liquid chromatographic (LC) and gas chromatographic (GC) methods used by these analytical laboratories for the detection and characterization of suspected illegal medicines and health products, including lifesaving drugs (antimicrobials and antimalarials), lifestyle drugs (erectile dysfunction drugs), and biotechnology drugs (doping peptides and skin-tanning peptides). Literature published from 2015 until early 2019 will be surveyed.
Over the past five years, incidents involving pharmaceutical crime increased by 60%, posing a growing threat to public health (1). Pharmaceutical falsification is a lucrative business. The profits of the illegal pharmaceutical market ranged from 75 billion to 200 billion US dollars in 2012, which resulted in the growing trend of pharmaceutical crime (2).
In order to come to a global uniform semantics, the World Health Organization (WHO) has adopted the term “substandard and falsified (SF) medical products’’, to represent three mutually exclusive classes, namely substandard medical products, unregistered or unlicensed medical products, and falsified medical products (3). Furthermore, the Falsified Medicines Directive 2011/62/EU will be executed in February 2019 to combat the threat of falsified medicines (4). The directive focuses on the prevention of falsified medical products from entering the legal supply chain of the European Union (EU) because obligatory safety features (a unique identifier and tamper-evident packaging) will be used to guarantee the authenticity of medical products. Moreover, additional strict rules are prescribed for the import of active substances and strengthened requirements of record-keeping for wholesale distributors will be put in place. Furthermore, internet pharmacies should display a common EU-wide logo on the website (4).
According to the WHO, SF medical products influence every region of the world (5). The International Criminal Policing Association (Interpol) estimated that an annual death toll of more than one million people was due to SF medical products (6). Recently, a survey on the available scientific peer-reviewed publications since 2006 demonstrated the occurrence of at least 48 nonrelated incidents that affected the health of thousands of adults and children worldwide, with a similar number of incidents in developing and developed countries (7). In the battle against SF medical products, it stands to reason that besides preventive strategies and stricter regulations and controls, efficient analytical methodologies are also paramount because they enable the surveillance of (illegal) medical products on the market. Chromatographic and hyphenated techniques allow a comprehensive analysis of SF medical products in terms of active pharmaceutical ingredients (APIs), impurities, and residual solvents, giving them a prominent role in the supervision of SF medical products.
Analytical Methods
The increased awareness of the potential dangers of SF medical products has resulted in numerous articles, in which chromatographic and spectroscopic techniques have been employed for the detection and characterization of SF medicines and illegal health products. In general, spectroscopic techniques, such as Fourierâtransform infrared spectrometry (FT-IR), near infrared spectroscopy (NIR), and Raman spectroscopy, are often used for a first evaluation or screening of suspected products, since they are fast and require less or no sample preparation. However, spectroscopic techniques are not always able to detect the presence of illicit APIs because of matrix interference, and they are of limited use for the quantification of APIs and the detection of impurities. Nuclear magnetic resonance (NMR) allows proper identification and quantification without utilization of standards, but this technique is rather complicated and requires milligrams of material. Nowadays, gas chromatography (GC) and liquid chromatography (LC) methods are the gold standards in analytical laboratories responsible for the analysis of SF medical products, despite the fact that reference substances are necessary for quantification. Identification issues can be tackled by hyphenation with mass spectrometry (GC–MSn or LC–MSn).
This article will discuss chromatographic techniques and their applications in lifesaving drugs (antimicrobials and antimalarial drugs), lifestyle drugs (erectile dysfunction drugs), chemical adulteration of dietary supplements, and biotechnological drugs.
Lifesaving Medicines: Antimicrobials and Antimalarials: According to the WHO, since 2013, antibiotics and antimalarials have been the most commonly reported SF medical products (8), and most are associated with African and Asian regions (9–13). Fadeyi et al. (10) and Frimpong et al. (12) investigated the quality of antimicrobials in Ghana by using LC–UV for the determination of the API content. More than half of the samples purchased in Ghana were found to be of poor quality. Recently, Islam et al. (14) evaluated the quality of antimicrobial drugs in Myanmar using pharmacopoeia methods (LC–UV). Out of 177 samples, 36 (20.3%) failed the assay tests. The use of LC–UV for the analysis of SF antimicrobials is more common in Africa because of limited access to sophisticated equipment.
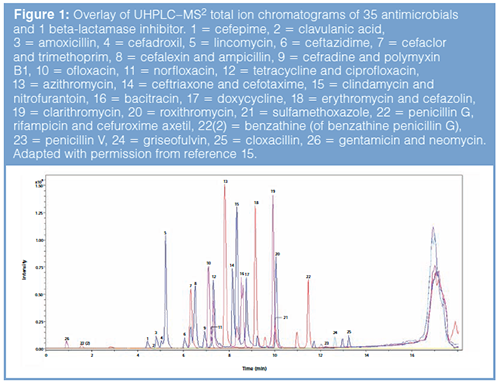
SF antimicrobials are also gaining in popularity in developed countries. In a very recent work of the authors, chromatographic methods were developed for the screening and subsequent quantification of suspected illegal antimicrobial drugs encountered on the Belgian market (15). Taking advantage of the additional selectivity of MS, a short ultrahighâpressure liquid chromatographic (UHPLC)–MS2 method was able to detect 35 antimicrobials and one betaâlactamase inhibitor (Figure 1). A longer UHPLC–diode array detection (DAD) method was applied to quantify the 35 antimicrobials and one betaâlactamase inhibitor. The results showed that half of the collected samples were noncompliant with the regulation. In this case, DAD was preferred over 3D ion trap MS for quantification because the former provides accurate results without the need for expensive isotopically labelled internal standards.
Antimalarials are of the utmost importance as lifesaving drugs. As stated by the WHO, half of the global population was at risk of malaria in 2016 (16). It has been reported that SF-antimalarial medicines are prevalent in Africa and Southeast Asia (9,13,17,18). In the past, often thin-layer chromatography (TLC) was used in these regions for the quality evaluation of antimalarials (19) because it is a cheap and easy technique, but, more recently, LC methods have also been applied (9,13,17). Generally, LC–MS is used to confirm claimed APIs and determine potential impurities, while LC–UV/DAD is used for subsequent quantification. For example, Kaur et al. (17) used LC–DAD and MS to find out that falsified artemisininâcontaining antimalarial samples contained no labelled API, but other potentially deleterious compounds, such as bis (2-ethylhexyl) adipate, dioctyl adipate, chlorzoxazone, ciprofloxacin, and acetaminophen.
Lifestyle Medicines: Erectile Dysfunction Drugs: Falsification of erectile dysfunction drugs is widespread in developed countries. LC, GC, and hyphenated techniques have been proposed for the surveillance of this illegal market. Lee et al. (20) developed a simple, fast, and selective LC–quadrupole time-of-flight (QTOF)-MS method for the screening of 40 analogues of sildenafil and the creation of a spectral library, including accurate mass, ion fragmentation patterns, and retention times. This powerful LC–QTOF-MS-based spectral library provided comprehensive and accurate mass data, which was necessary and useful in the analysis of complex SF erectile dysfunction drugs. A case report described that Kamagra tablets (containing sildenafil, but not registered in the EU) seized by the German police contained large amounts of 2-mercaptobenzothiazole (MBT), which is known as a human contact allergen and a rubber vulcanizing accelerator. High-resolution UHPLC–orbital trap MS2 was used for the identification of sildenafil and the detection of MBT, although the initial screening using handheld FT-IR gave a negative result (21). Subsequent quantifications were achieved by using the LC–DAD method described in the European Pharmacopoeia (22). With regard to quantification, Fidan et al. (23) developed and validated a LC–UV method to determine simultaneously sildenafil and tadalafil in SF erectile dysfunction drugs within 6 min. The method was fast, but was unable to detect impurities. For the detection and identification of analogues and impurities, a technique for compound structure elucidation is required, for example, high-resolution (HR)MS and NMR.
Although most of the studies on the evaluation of erectile dysfunction drugs use LC systems because of the high boiling point and high molecular weight of these drugs, Jeong et al. (24) applied high-temperature GC–MS (HTGC–MS) for the simultaneous determination of sildenafil, tadalafil, and vardenafil in erectile dysfunction drugs. Full scan mode and an internal standard were used to obtain an efficient qualitative and quantitative method for routine analysis. HTGC–MS generated improved peak shapes and provided faster analysis compared to conventional GC. Moreover, HTGC–MS could avoid matrix effects. Concerning the in-field evaluation of SF erectile dysfunction drugs, a portable GC–MS system was introduced to analyze residual solvents (25).
Chemical Adulteration of Dietary Supplements: Since dietary supplements are sold directly to consumers and regulations are not as strict as for pharmaceuticals, they are vulnerable to adulteration. Many types of adulterants have been reported in food supplements. Commonly, the majority of adulterants found in dietary supplements can be classified as sports performance enhancers (for example, anabolicâandrogenic steroids [AAS]), weight loss enhancers (for example, sibutramine with or without antidepressant), and sexual performance enhancers (for example, phosphodiesterase type 5 inhibitor [PDE-5i]) (26).
The use of UHPLC–MS/MS for target screening and quantification of 26 different AAS in food supplements was reported by Paíga et al. (27) and Cho et al. (28). Internal standards were employed by Paíga et al. to quantify the adulterants, whilst Cho et al. used the most sensitive product ion for quantification. In addition, Neves et al. (29) presented a GC–MS method for the identification and quantification (using internal standards) of 13 AAS in medicines and dietary supplements. Seventeen dietary supplements from the black market were analyzed, of which five contained unlabelled AAS at a pharmacological relevant quantity. The use of an internal standard for quantification can solve problems caused by matrix effects. All of the above-mentioned methods require sample cleanup procedures; however, Xia et al. (30) proposed a rapid screening method of SF slimming capsules without sample pretreatment using an approach based on the electronic nose and flash GC combined with chemometrics. This method was able to discriminate slimming capsules to which sibutramine and phenolphthalein had been illegally added. For the adulterants found in dietary supplements for sexual performance enhancement, GC–MS (31) and UHPLC–QTOF-MS (32,33) were described for the screening. Moreover, Kim et al. (32) extended the UHPLC–QTOF-MS full-scan screening method for erectile dysfunction drugs to the detection of multiclass illegal adulterants, including synthetic steroids, anabolic steroids, and antihistamine drugs. Six out of 70 samples analyzed were found to be positive for illegal adulterants. Taken together, these reports clearly demonstrate the utility and importance of GC or LC coupled to MS for the detection of adulterants in dietary supplements.
It has been reported that various other classes of adulterants were detected in dietary supplements, that is, diuretics, antidiabetics, cognition enhancers (amphetamine or analogues and vinpocetine), and synthetic hair-growth drugs (34–37). Ki et al. (34) utilized LC–QTOF-MS to screen 35 diuretics and antidiabetics using an in-house library. Avula et al. (35) used UHPLC–DAD to identify and quantify vinpocetine and picamilon in dietary supplements sold in the United States and never approved by the US Food and Drug Administration.
However, over the past five years, it has been reported that some new PDE-5i analogues were detected in dietary supplements (38). The identification of these new analogues is more challenging, since their structures are unknown. This often requires an approach using multiple techniques. Generally, most studies employed HRMS or NMR techniques for structure elucidation. Kern et al. (39) and Yun et al. (40) identified a new tadalafil analogue and a new sildenafil analogue, respectively, in dietary supplements. They both used LC–DAD to isolate the unknown target and evaluated the compound via the UV spectrum. However, while Yun et al. confirmed the structure with the synthesized reference using LC–QTOF-MS, NMR, and FT-IR, Kern et al. used LC–HRMS and GC–FT-IR–MS for structure elucidation. For another case, in which a certified reference was unavailable, Vanhee et al. (26) disclosed the presence of adrafinil (a synthetic cognition enhancer) in food supplements utilizing GC–MS, LC–MS2, and NMR analysis.
Peptide and Biotechnology Drugs: In recent years, a new type of unregistered or unlicensed drug has emerged in the form of peptides, which are gaining popularity as demonstrated by Venhuis et al. (41). In order to identify the substances present in that type of sample, either NMR or LC–MS are utilized.
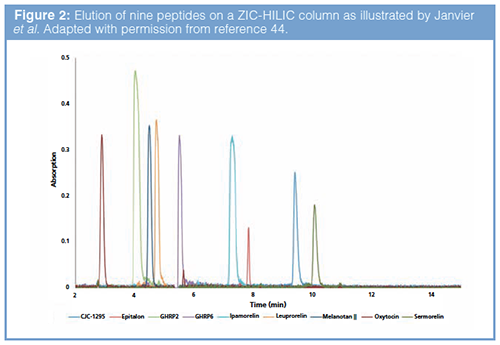
Gaudiano et al. (42) applied both high-resolution LC–MS and NMR to identify GHRP-2, an unauthorized synthetic peptide used predominantly for illegal doping purposes. In addition to doping peptides, skin-tanning peptides, potential anti-ageing peptides (for example, epitalon), and potential cognition-enhancing peptides have been encountered (43,44,26). Vanhee et al. (43) developed and validated a reversedâphase LC–MS2 method to identify illegal peptide biopharmaceuticals, extended with a subsequent quantification method via UHPLC–DAD. Although quantification via LC–MS2 is more sensitive, in this case LC–UV was sufficient to quantify the amount of peptides in illegal products. The method was able to selectively detect 25 peptides and was applied to analyze 65 suspected illegal samples confiscated by the Belgian Federal Agency for Medicines and Health Products (FAMHP). The analytical results disclosed that almost all collected illegal peptides were underdosed and the most frequently detected illicit peptides were doping agents. However, by applying this reversed-phase LC method, some more polar peptides (epitalon and protirelin), which eluted at the very beginning of the gradient, could not be quantified. In addition, GHRP-2 coeluted with leuprorelin. To solve these problems, Janvier et al. (44) envisaged an alternative separation strategy, based on hydrophilic interaction chromatography (HILIC) (Figure 2), without any need for sample preparation. The aforementioned reversed-phase LC method combined with the HILIC method allowed the routine analysis of illegal peptide drugs frequently encountered by controlling agencies. In another case study, the polypeptide epidermal growth factor (EGF) was found in an illegal injection sample that was labelled to contain insulin-like growth factor-1 (IGF-1) (45). EGF can bind to the EGF-receptor and possibly promote tumour cell motility, posing potential danger to consumers. The presence of EGF was identified by screening with low-resolution LC–MS2 techniques and subsequent confirmation using LC–HRMS (45). It is evident that HRMS devices play a pivotal role in the identifications of unknown peptide drugs.
In addition to peptide drugs, biotech drugs, in the form of proteins, including monoclonal antibodies (mAbs), are also gaining in popularity. Although until recently only doping proteins (for example, human growth hormone, erythropoietin, human chorionic gonadotropin) were encountered, SF mAbs have been found across the globe. LC–MSn is once again the preferred methodology, upon sample pretreatment (for example, digestion with trypsin), for the characterization of large biotherapeutics (46).
Conclusion
This review gives a general overview of the literature published from 2015 on the application of LC and GC methods for the analysis of popular SF medicines and health products. In general it can be said that LC and GC are able to tackle the majority of the challenges encountered during the analysis of a broad range of SF medicines and illegal dietary supplements. These chromatographic methods display several advantages of high sensitivity and accuracy in terms of API and impurity (either API- or process-related) or contamination analysis. APIs, potential impurities, and excipients are first separated through LC or GC and then detected and quantified using mainly UV and different modes of MSn for further identification and structure elucidation. It should be noted that techniques like LC and GC require reliable reference substances to perform proper quantification.
The Falsified Medicines Directive 2011/62/EU will be implemented in February 2019, and pharmaceutical manufacturers and associated supply chain distributors will have to comply. Tamper-evident packaging techniques will be developed to curtail the manufacturing, distribution, and supply of SF medicines and illegal health products. In this context, the drug tablet film can be coded. LC and GC could be used in the coding analysis of tablet coating to protect genuine drug products from falsification or adulteration. For example, Ilko et al. (47) proposed a novel strategy of adding monodisperse polyethylene glycol (PEG) into tablet coating solutions to create a code on individual tablet film. These writing codes could be easily recognized and confirmed through LC–MSn analysis. With the advances in LC and GC, analytical methods are continuously being developed and optimized for in-depth scrutiny of SF medical products and illegal dietary supplements.
References
- PSI, Pharmaceutical Security Institute, (2018). http://www.psi-inc.org/geographicDistributions.cfm (accessed 30 October 2018).
- World finance, Trade in illegal medicine hits pharmaceutical sector, (2012). https://www.worldfinance.com/special-reports/trade-in-illegal-medicine-hits-pharmaceutical-sector (accessed 30 October 2018).
- World Health Organization, WHO global surveillance and monitoring system for substandard and falsified medical products, Geneva (2017). http://www.who.int/medicines/regulation/ssffc/publications/GSMS_Report.pdf?ua=1.
- European Commission, Falsified Medicines Directive 2011/62/EU, (2018). https://ec.europa.eu/health/human-use/falsified_medicines_en (accessed 12 December 2018).
- World Health Organization, Substandard and falsified medical products, (2018). http://www.who.int/news-room/fact-sheets/detail/substandard-and-falsified-medical-products (accessed 6 September 2018).
- N. Southwick, Counterfeit drugs kill 1 Mn people annually: interpol, InSight Crime, (2013). https://www.insightcrime.org/news/brief/counterfeit-drugs-kill-1-million-annually-interpol/ (accessed 31 October 2018).
- M.S. Rahman, N. Yoshida, H. Tsuboi, N. Tomizu, J. Endo, O. Miyu, Y. Akimoto, and K. Kimura, Trop. Med. Int. Heal. 23, 1294–1303 (2018).
- World Health Organization, Substand and falsified medical products, (2018). http://www.who.int/mediacentre/factsheets/fs275/en/(accessed 31 October 2018)
- H. Kaur, E.L. Allan, I. Mamadu, Z. Hall, M.D. Green, I. Swamidos, P. Dwivedi, M.J. Culzoni, F.M. Fernandez, G. Garcia, D. Hergott, and F. Monti, BMJ Glob Health2, e000409 (2017).
- I. Fadeyi, M. Lalani, N. Mailk, A. Van Wyk, and H. Kaur, Am. J. Trop. Med. Hyg.92, 87–94 (2015).
- N. Alotaibi, S. Overton, S. Curtis, J.W. Nickerson, A. Attaran, S. Gilmer, and P.M. Mayer, Am. J. Trop. Med. Hyg.99, 477–481 (2018).
- G. Frimpong, K. Ofori-Kwakye, N. Kuntworbe, K.O. Buabeng, Y.A. Osei, M. El Boakye-Gyasi, and O. Adi-Dako, J. Trop. Med.2018, 1–14 (2018).
- S. Yeuchaixiong, H. Kaur, P. Tabernero, S. Sengaloundeth, I. Swamidoss, M.D. Green, F.M. Fernández, M. Khanthavong, P. Dwivedi, M. Mayxay, C. Vilayhong, E.L. Allan, P.N. Newton, M.J. Culzoni, C. Phonlavong, and C. Sichanh, Am. J. Trop. Med. Hyg. 92, 95–104 (2015).
- M. Islam, N. Yoshida, K. Kimura, C. Uwatoko, M. Rahman, S. Kumada, J. Endo, K. Ito, T. Tanimoto, T. Zin, and H. Tsuboi, Pharmacy6, 96 (2018).
- Y. Tie, C. Vanhee, E. Deconinck, and E. Adams, Talanta194, 876–887 (2019).
- World Health Organization, 10 facts on malaria, (2016). http://www.who.int/features/factfiles/malaria/en/ (accessed 22 November 2018).
- H. Kaur, E.L. Allan, I. Mamadu, Z. Hall, O. Ibe, M. El Sherbiny, A. Van Wy, S. Yeung, I. Swamidoss, M.D. Green, P. Dwivedi, M.J. Culzoni, S. Clarke, D. Schellenberg, F.M. Fernández, and O. Onwujekwe, PLoS One10, 1–13 (2015).
- J.P. Mufusama, K. Ndjoko Ioset, D. Feineis, L. Hoellein, U. Holzgrabe, and G. Bringmann, Drug Test. Anal.10, 1599–1606 (2018).
- M. Lalani, F.E. Kitutu, S.E. Clarke, and H. Kaur, Malar. J.16, 1–14 (2017).
- S. Lee, D. Ji, M. Park, and K.H. Chung, Forensic Sci. Int. 257, 182–188 (2015).
- P.H.J. Keizers, A. Wiegard, and B.J. Venhuis, J. Pharm. Biomed. Anal. 131, 133–139 (2016).
- European Pharmacopoeia, 9th ed. (European Directorate for the Quality of Medicines, Strasbourg, France, 2018).
- A.K. Fidan and S. Bakirdere, J. AOAC Int.99, 923–928 (2016).
- Y.D. Jeong, S.I. Suh, J.Y. Kim, M.K. In, and K.J. Paeng, Chromatographia79, 1671–1678 (2016).
- P.E. Leary, G.S. Dobson, and J.A. Reffner, Appl. Spectrosc.70, 888–896 (2016)
- C. Vanhee, E. Tuenter, A. Kamugisha, M. Canfyn, G. Moens, P. Courselle, L. Pieters, E. Deconinck, and V. Exarchou, J. Forensic Toxicol. Pharmacol. 7, 1–7 (2018).
- P. Paíga, M.J.E. Rodrigues, M. Correia, J.S. Amaral, M.B.P.P. Oliveira, and C. Delerue-Matos, Eur. J. Pharm. Sci.99, 219–227 (2017).
- S.H. Cho, H.J. Park, J.H. Lee, J.A. Do, S. Heo, J.H. Jo, and S. Cho, J. Pharm. Biomed. Anal. 111, 138–146 (2015).
- D.B.D Neves and E.D. Caldas, Forensic Sci. Int.275, 272–281 (2017).
- Z. Xia, W. Cai, and X. Shao, J. Sep. Sci.38, 621–625 (2015).
- S.U. Mokhtar, S.T. Chin, C.L. Kee, M.Y. Low, O.H. Drummer, and P.J. Marriott, J. Pharm. Biomed. Anal.121, 188–196 (2016).
- E.H. Kim, H.S. Seo, N.Y. Ki, N.H. Park, W. Lee, J.A. Do, S. Park, S.Y. Baek, B. Moon, H. Bin Oh, and J. Hong, J. Chromatogr. A1491, 43–56 (2017).
- X.B. Wang, J. Zheng, J.J. Li, H.Y. Yu, Q.Y. Li, L.H. Xu, M.J. Liu, R.Q. Xian, Y.E. Sun, and B.J. Liu, J. Food Drug Anal.26, 1138–1153 (2018).
- N.Y. Ki, J. Hur, B.H. Kim, K.H. Kim, B.J. Moon, H. Bin Oh, and J. Hong, J. Food Drug Anal., in press (2018).
- B. Avula, A.G. Chittiboyina, S. Sagi, Y.H. Wang, M. Wang, I.A. Khan, and P.A. Cohen, Drug Test. Anal.8, 334–343 (2016).
- J.H. Lee, G. Kang, H.N. Park, J. Kim, N.S. Kim, S. Park, S.K. Park, S.Y. Baek, and H. Kang, Food Addit. Contam.-Part A35, 191–199 (2018).
- P.A. Cohen, C. Bloszies, C. Yee, and R. Gerona, Drug Test. Anal.8, 328–333 (2016).
- C.L. Kee, X. Ge, V. Gilard, M. MaletâMartino, and M.Y. Low, J. Pharm. Biomed. Anal.147, 250–277 (2018).
- S.E. Kern, L.M. Lorenz, A. Lanzarotta, E.A. Nickum, and J.J. Litzau, J. Pharm. Biomed. Anal.128, 360–366 (2016).
- J. Yun, K.J. Shin, J. Choi, K. Kwon, and C.H. Jo, J. Chromatogr. B1072, 273–281 (2018).
- B.J. Venhuis, P.H.J. Keizers, R. Klausmann, and I. Hegger, Drug Test. Anal. 8, 398–401 (2016).
- M.C. Gaudiano, L. Manna, M. Bartolomei, A.L. Rodomonte, P. Bertocchi, E. Antoniella, L. Romanini, S. Alimonti, L. Rufini, and L. Valvo, Ann Ist Super Sanità 52, 128–132 (2016).
- C. Vanhee, S. Janvier, B. Desmedt, G. Moens, E. Deconinck, J.O. De Beer, and P. Courselle, Talanta142, 1–10 (2015).
- S. Janvier, E. De Sutter, E. Wynendaele, B. De Spiegeleer, C. Vanhee, and E. Deconinck, Talanta174, 562–571 (2017).
- C. Vanhee, S. Janvier, G. Moens, S. Goscinny, P. Courselle, and E. Deconinck, Drug Test. Anal.9, 831–837 (2017).
- S. Janvier, B. De Spiegeleer, C. Vanhee, and E. Deconinck, J. Pharm. Biomed. Anal. 161, 175–191 (2018).
- D. Ilko, C. Steiger, R. Keller, U. Holzgrabe, and L. Meinel, Eur. J. Pharm. Biopharm.99, 1–6 (2016).
Yaxin Tie ââis currently a Ph.D. student at the KU Leuven and Sciensano in Brussels, Belgium. Her research work focuses on the characterization and hazard identification of substandard and falsified antimicrobials encountered in Europe.
Celine Vanhee has a Ph.D. in biochemistry and works for the Belgian official medicines control laboratory, part of Sciensano, Brussels. She is, together with Eric Deconinck, responsible for the (bio)chemical analysis of falsified medicines, including peptide drugs and biotherapeutics.
Erwin Adams is a professor at the KU Leuven, Belgium. He performs research on pharmaceutical analysis with a focus on analytical method development and validation for quality control of medicines.
Eric Deconinck has a Ph.D. in pharmaceutical sciences and is currently head of the scientific service Medicines and Health Products of Sciensano. This service acts as the Belgian Official Medicine Control Laboratory and National Reference Laboratory for the quality control of medicines. His research interests are mainly in the analysis and risk evaluation of illegal medicines and health products.
Deirdre Cabooter is the editor of “Pharmaceutical Perspectives”. She is an associate professor at the Department of Pharmaceutical and Pharmacological Sciences of KU Leuven, in Leuven, Belgium. She is also a member of LCGC Europe’s editorial advisory board. Direct correspondence about this column to the editor-in-chief, Alasdair Matheson, at alasdair.matheson@ ubm.com

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.









